
Nikhil Gangavane/Dreamstime.com
This content was published in 2010. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
Delirium occurs in a wide variety of healthcare settings. It is an acute onset mental disorder characterised by a reduced ability to focus, sustain or shift attention, in combination with a change in cognition or the development of a perceptual disturbance.
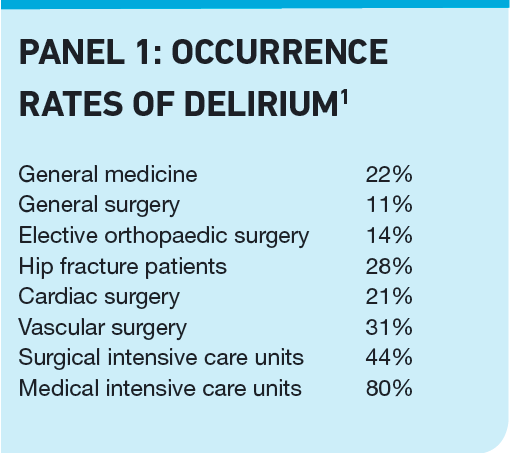
Acutely ill patient populations suffer the greatest incidence of delirium, but the condition is common even in long-term care institutes, with a 16 per cent rate of occurrence. Panel 1 lists occurrence rates in other areas of healthcare.1
Symptoms and detection
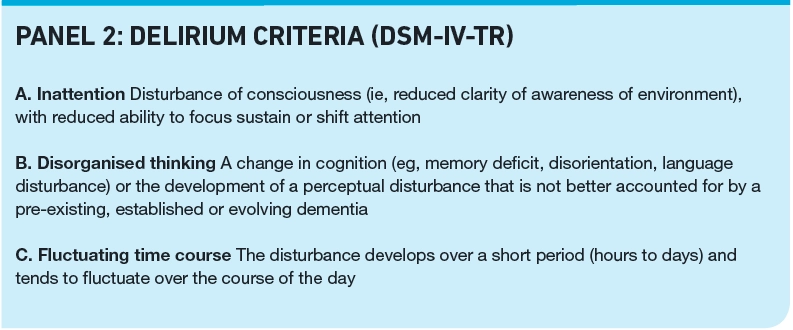
The symptoms and severity can fluctuate over hours to days. Panel 2 lists the American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV-TR) criteria for diagnosis of delirium.
The disorder is most often recognised when patients draw attention to themselves by combative, aggressive or bizarre behaviour. This type of delirium has been termed “hyperactive delirium”, with the adjective depicting the excessive motor activity seen. Healthcare professionals tend to focus on these cases, even though they do not form the bulk of patients with delirium. Some delirious patients, however, become withdrawn, quiescent and listless, and are said to suffer from hypoactive delirium. These patients can be labelled as being “pleasantly confused” or as suffering from depression. Patients with a third motoric type (mixed delirium) fluctuate between displaying hyperactive and hypoactive symptoms. In a palliative care study, 14 per cent of delirious patients were found to have hyperactive delirium2 and in a critical care study, only 1.6 per cent of patients had pure hyperactive delirium, 43.5 per cent had hypoactive delirium and the remaining 54.9 per cent had mixed delirium.3
Recent literature describes a fourth group in older patients: delirious with normal psychomotor activity.4 A study found that 20 per cent of delirious patients display some form of hyperactive motor activity (pure hyperactive group plus mixed group), 30 per cent display pure hypoactive delirium and the remaining 50 per cent have normal motor activity. Healthcare professionals who rely on the presence of agitated behaviours before considering a diagnosis of delirium could easily miss the condition in many patients.
Appropriate delirium detection and management is crucial to improve patient experience and outcomes. Delirium is often unrecognised in patients admitted to general wards and the behavioural consequences that arise from the condition may cause the patient to be labelled as “troublesome”. Carers can play an important role in the community to support early detection and because community pharmacists may be the first port of call for the carer, it is important for them to recognise symptoms in order to signpost carers towards the appropriate support. Pharmacists can ask four questions if delirium is suspected:
- Is there a problem with attention? (This could mean the person is inattentive and does not focus or that he or she can focus on tasks but at the expense of all else — failure to shift attention.)
- Is thinking disorganised (such as not being able to remember things or being confused)?
- Is there an altered level of conciousness (hyper-alertness and on edge, or sluggish, drowsy or withdrawn)?
- Is the change sudden or does it fluctuate over hours or days?
Other signs and symptoms of delirium include sleep-wake cycle disturbance (eg, sleeping during the day and being up at night), perceptual disturbances (eg, hallucinations, illusions), emotional disturbances and delusions. Referral in primary care would first be to GPs. The patient should be seen urgently (whether he or she lives in their own home or in a care home). Where patients’ behaviour poses a risk to themselves or others, or is significantly altered from normal, they should be directed to an emergency department or an ambulance should be called.
Several screening tools have been validated to identify patients with features of delirium. The CAM (confusion assessment method) instrument is recommended by the National Institute for Health and Clinical Excellence for general use and a modified form, CAM-ICU, can be used in critical care.
A diagnosis is made if the patient meets the DSM-IV-TR criteria. Baseline cognitive function may not be known and can be low (eg, in dementia). If collateral history is not available, making the diagnosis of delirium can be difficult. Carers will be able to report preexisting dementia or highlight any acute changes in cognitive function. The wider multi-professional team (pharmacists, physiotherapists, etc) might notice acute changes in cognition and so be able provide evidence that supports a diagnosis.
Reflect
- How is delirium diagnosed?
- How can delirium be prevented?
- What treatment options are available?
Before reading on, think about how this article may help you to do your job better.
Causes, risk factors and outcomes
Delirium can be caused by a wide variety of insults, but there are general themes. The physiological consequences of a medical condition, such as a severe infection, are a common cause. Medication can be causative or contributory through intoxication, unintended side effects, or through drug withdrawal syndromes. Alcohol withdrawal can also produce delirium.
Pre-existing dementia is a major risk factor for delirium. (In addition, it poses a particular problem when older people are admitted to general medical and surgical wards, which have less experience with dementia, rather than specialist elderly care wards.) According to NICE, other high risk factors are:
- Being aged 65 years or over
- Having a hip fracture
- Having a clinical condition that is deteriorating or that is at risk of deterioration
The neurochemical pathophysiology of delirium is poorly understood but the hypothesis of a disturbance in a final common pathway involving cholinergic and dopaminergic systems is widely accepted. Insults associated with delirium formation generally reduce cholinergic tone or increase dopaminergic activity, either directly, or through interacting systems. Pharmacological strategies to treat delirium have tended to target cholinergic or dopaminergic systems.
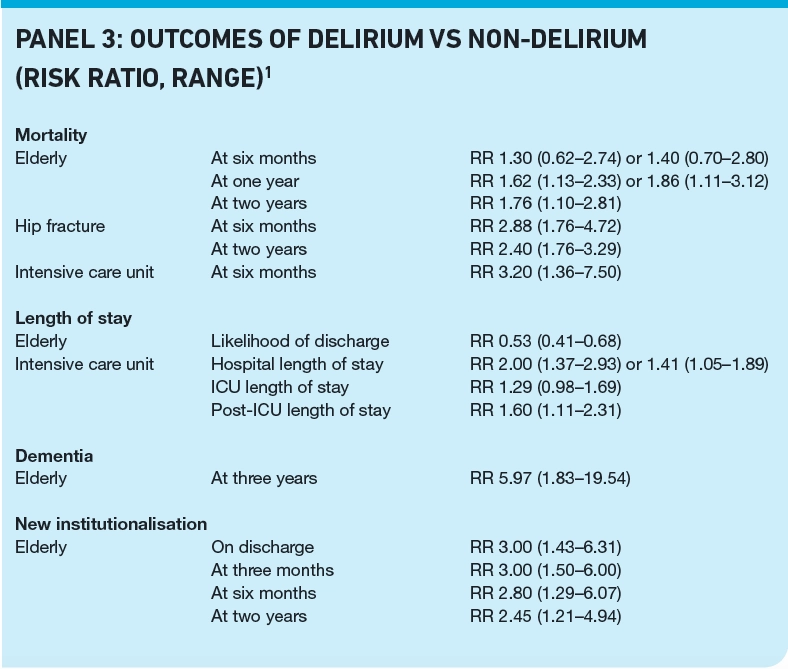
Patients with delirium are at an increased risk of adverse outcomes compared with patients without delirium. These risks appear to be greater in some patient groups. For example, elderly patients with delirium are 1.3 to 1.4 times more likely to die six months after their discharge compared with non-delirious patients, and delirious critically ill patients are 3.2 times more likely to die. Panel 3 summarises risks of delirium for mortality and other adverse outcomes in different patient groups.1 In addition to the effect on objective endpoints, delirium is extremely distressing and frightening for patients, carers and relatives.8
Large numbers of patients across primary and secondary care are affected by delirium so the potential benefits in avoiding or effectively treating the condition are huge, especially if delirium causes the associated adverse outcomes and is not merely a marker for them — there is evidence that reducing delirium rates has a beneficial effect on some of these outcomes.
Prevention
The first step in prevention is timely and appropriate use of non-pharmacological methods by systematic application of good quality healthcare. Panel 4 gives examples of these methods. Many are common sense, such as addressing poor nutrition, dehydration and sensory impairments (eg, encouraging use of correct glasses and working hearing aids).

NICE recently analysed non-pharmacological interventions. The cost of the intervention (eg, staff and materials) was outweighed by savings of £8,730 per quality-adjusted life year, which was predominantly achieved through reduced dementia (in some patients prolonged delirium may be followed by dementia), need for new institutionalisation and length of hospital stay.2 Multicomponent intervention studies have been found to reduce delirium rates and NICE recommends a programme of interventions targeted at patients at high risk in either primary or secondary care institutes.1
Medication
Pharmacists have a large role to play in identifying medication that can increase the risk of delirium. Once identified, a plan that includes switching to alternatives or reducing drug doses can be implemented where possible. Examples of medicines that can cause or exacerbate an existing delirium are given in Panel 5. Such drugs affect dopaminergic and cholinergic systems, either directly, or through interacting pathways (eg, GABAminergic, glutamatergic, serotonergic).

Depression of cholinergic systems is thought to be a major cause of drug induced delirium and it has been suggested that the deliriogenic potential of some medicines is linked to anticholinergic potency. It should also be noted that some drugs, such as digoxin, nifedipine and prednisolone, possess a degree of anticholinergic activity that might not be recognised by healthcare professionals.
A study that included a clinical medication review to minimise drug triggers showed reduced delirium rates and a large reduction in length of hospital stay in the intervention group (9.4 days vs 13.4 days, P=0.001) as well as reduced mortality (2 vs 9, P=0.03, n=400).5 Pharmacists can perform medication reviews and offer advice on timing of medications. For example, sedatives should not be given during the day, which can sometimes happen if a carer’s evening visit, which includes medication, is at 5pm.
Pharmacological prophylaxis
Several studies have been conducted using antipsychotic or cholinergic agents in an effort to prevent patients at high risk of delirium from transitioning into delirium. In general, the effectiveness of cholinergic drugs (such as donepezil or rivastigmine) for prophylaxis has been disappointing.
The use of antipsychotic medicines yields more encouraging results. One study used small doses of haloperidol (0.5mg three times daily) in elderly hip fracture patients but showed no reduction in delirium rates. There was, however, a reduction in duration of delirium, severity of delirium and a substantial reduction in hospital length of stay of 5.5 days (17.1 days vs 22.6 days, P<0.001).6 Another study found that giving 5mg haloperidol intravenously at night for five nights after gastrointestinal surgery reduced the rate of delirium from 32.5 per cent to 10.5 per cent (P<0.05)7 and a study in post cardiac patients found that a single dose of risperidone 1mg sublingually on emergence from anaesthesia significantly reduced delirium rates postoperatively (11.1 per cent vs 31.7 per cent, P=0.009).8
The prophylactic use of antipsychotics, however, is highly controversial. NICE does not recommend prophylactic antipsychotics, but the targeted use of prophylaxis to patient groups at high risk of delirium is the subject of ongoing research. Prophylaxis must be balanced against the risks of overuse of antipsychotic drugs in older people, which have been highlighted in Department of Health documents and in the media, and the potential for unintended continuation of medication after clinical need has passed.
Treatment
Despite the use of preventive strategies to reduce delirium rates, significant numbers of patients still transition into delirium and require further management.
Antipsychotics are considered first-line therapy for established delirium (although benzodiazepines should be used where the delirium is secondary to alcohol withdrawal). The optimum doses are not known and research in this area is lacking —there are only two controlled studies in the literature.
One large study compared haloperidol with olanzapine versus a control arm in older patients with delirium.9 Olanzapine was started at 1.25–2.5mg daily orally or sublingually and titrated to 1.25mg–20mg daily depending on symptoms. Haloperidol was started at 2.5mg–10mg intramuscularly per day.
The delirium rating scale (DRS), a validated delirium scoring system, was used to assess delirium severity on a daily basis. The DRS scores in the haloperidol and olanzapine groups were significantly less than control from day 1 to the end of the study on day 7 (P<0.01), olanzapine took effect faster than haloperidol and both were faster than control (2.8 days vs 3.4 days vs 5.2 days).
The average dose of haloperidol needed to take effect was 7.1mg per day and for olanzapine this was 4.5mg per day. The number needed to treat for complete delirium resolution after seven days of therapy was 2 for haloperidol and 3 for olanzapine.
A recent pilot study compared the effectiveness of quetiapine 50mg twice daily (oral or nasogastric) with placebo in critically ill patients.10 If rescue haloperidol was required, the quetiapine dose (or placebo equivalent) was increased in increments of 50mg 12 hourly to a maximum of 200mg twice daily. The intensive care delirium screening checklist (ICDSC) is a validated score for delirium in critically ill patients and was used to assess delirium. During the study, delirium resolved at least once in all patients receiving quetiapine and in 78 per cent (14 of 18) patients receiving placebo (P=0.05). Quetiapine patients spent fewer hours in delirium (36h versus 120h, P=0.006) and required a shorter duration of study drug therapy (102h versus 186h, P=0.04). There was no significant difference in rescue haloperidol dose between groups, but the duration of haloperidol therapy was shorter in the quetiapine group. Dose escalation was less in the quetiapine group compared with the placebo group (200mg twice daily versus 375mg equivalent twice daily, P=0.02).
There are suggestions that the different psychomotor forms of delirium may respond to different types of antipsychotic although the evidence base for this is weak. Hypoactive delirium may respond better to atypical antipsychotics such as risperidone or aripiprazole. If this is borne out in larger studies it will be an important finding. Older patients tend to exhibit hypoactive delirium which is more common overall than hyperactive delirium and has been shown to have worse outcomes. It is too early to definitively recommend atypicals over typical antipsychotics for hypoactive delirium.
Pharmacists should ensure that the pharmacological management of delirium is pursued for as short a time as possible, discontinuing medication as symptoms resolve. Particular care is needed because of the link between delirium and dementia — it should be noted that the Medicines and Healthcare products Regulatory Agency has issued warnings about the consequences of over-prescribing antipsychotics in dementia patients.
Conclusions
Healthcare professionals are beginning to recognise the importance of delirium and realise that this common condition is not just a normal consequence of illness, but an aberrant response that, if left uncontrolled, has serious short- and long-term consequences for patients. Although a rich evidence base is lacking, non-pharmacological and pharmacological therapeutic options are available that can treat the condition and improve the outcome for the patient.
Pharmacists in both primary and secondary care are well positioned to identify patients at risk of delirium, identify patients with symptoms suggestive of delirium and refer such patients where appropriate. Patients who are exhibiting new symptoms should be referred or highlighted to healthcare workers who are trained to manage delirium.
In secondary care institutes, pharmacists should ensure that the relevant medical team or service is made aware of the development of new symptoms suggestive of delirium. They also have a role in highlighting cases of delirium for senior doctor review (registrar level and above) or supporting nursing staff in doing so where junior doctors are struggling to treat delirious patients appropriately.
Pharmacists may also be involved in writing the medication-related aspect of delirium management protocols and NICE guidance should be used when doing so. Additional sources aimed at specific areas of practice or general reviews of medication therapy may be useful adjuncts because smaller trials, case series and case reports not included by NICE may be deemed useful.
Pharmacists can also advise on the management of risk factors, provide strategies to remove exacerbating drugs and provide advice on the pharmacological management of delirium.
Practice points
Reading is only one way to undertake CPD and the regulator will expect to see various approaches in a pharmacist’s CPD portfolio.
- Look for drugs that can cause delirium during medicines use reviews.
- Make sure your team is aware of the UKCPA guidance on delirium (see Further reading) as well as the new guidance from NICE.
- Discuss with your staff, how you can support carers who visit your pharmacy.
Consider making this activity one of your nine CPD entries this year.
Signposting
- Information for carers about delirium is available from the European Delirium Association (www.europeandeliriumassociation. com) and The Royal College of Psychiatrists (www.rcpsych.ac.uk).
Further reading
- Brown TM. Drug-induced delirium. Seminars in Clinical Neuropsychiatry 2000; 5:113–2.
- British Geriatrics Society. Guidelines for the prevention, diagnosis and management of delirium in older people in hospital. Available at www.bgs.org.uk.
- United Kingdom Clinical Pharmacy Association. Detection, prevention and treatment of delirium in critically ill patients. Available at www.ukcpa.org.
Resources
- The confusion assessment method tool can be accessed at http://elderlife.med.yale.edu.
References
- The confusion assessment method tool can be accessed at http://elderlife.med.yale.edu. References 1 Delirium: diagnosis, prevention and management. National Institute for Health and Clinical Excellence, June 2010.
- Spiller JA, Keen JC. Hypoactive delirium: assessing the extent of the problem for inpatient specialist palliative care. Palliative Medicine 2006;20:7–23.
- Peterson JF, Pun BT, Dittus RS, Thomason JW, Jackson JC, Shintani AK et al. Delirium and its motoric subtypes: a study of 614 critically ill patients. Journal of the American Geriatrics Society 2006;54:479–84.
- Yang FM, Marcantonio ER, Inouye SK, Kiely DK, Rudolph JL, Fearing MA et al. Phenomenological subtypes of delirium in older persons: patterns, prevalence and prognosis. Psychosomatics 2009;50:248–54.
- Lundstrom M, Edlund A, Karlsson S, Brannstrom B, Bucht G, Gustafson Y. A multifactorial intervention program reduces the duration of delirium, length of hospitalisation and mortality in delirious patients. Journal of the American Geriatrics Society 2005;53:622–62.
- Kalisvaart KJ, De Jonghe JFM, Bogaards MJ Vreeswijk R, Egberts TC, Burger BJ et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. Journal of the American Geriatrics Society 2005;53:1658–66.
- Kaneko T, Cai J, Ishikura T, Kobayashi M, Naka T, Kaibara N. Prophylactic consecutive administration of haloperidol can reduce the occurrence of postoperative delirium in gastrointestinal surgery. Yonago Acta Medica 1999;42:179–84.
- Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesthesia and Intensive Care. 2007;35:714–9.
- Hu H, Deng W, Yang H, Liu Y. Chin J. Olanzapine and haloperidol for senile delirium: a randomized controlled observation. Clinical Rehabilitation 2006;10:188–90.
- Devlin JW, Roberts RJ, Fong JJ, Skrobik Y, Riker RR, Hill NS et al. Efficacy and safety of quetiapine in critically ill patients with delirium: a prospective, multicenter, randomized, double-blind, placebo-controlled pilot study. Critical Care Medicine 2010;38:419–27.
CPD articles are commissioned by The Pharmaceutical Journal and are not peer reviewed.


