This content was published in 2006. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
Vaccines prevent death on a large scale, but only if enough people are immunised. If most of the people within a population are vaccinated against an infection, those who are not will still be protected because spread of the pathogen is blocked. This is known as “herd immunity”. However, this immunity relies on public support of vaccination programmes which, in turn, strongly depends on perception of risk versus benefit. For example, the controversy relating to the safety of the combined measles, mumps and rubella (MMR) vaccine stemming from proposed links with autism has had a detrimental impact on the protection of children from these diseases within the UK. While most of the researchers who initially proposed this link subsequently retracted their speculations, the damage in terms of public confidence was done. A marked reduction in the percentage of the population immunised against MMR (from 92 to 82 per cent) occurred across the UK, with some areas of London reporting an uptake 20 per cent lower than the national average.1 The World Health Organization recommends an immunity level of 95 per cent to prevent disease outbreaks. The reduction in MMR vaccinations has been reflected by increased reports of measles and mumps in the UK and, in response, some health authorities are holding publicity events and training primary care staff to encourage uptake of the combined vaccine.
Source: Mike Wyndham Medical Picture Library
Basic principles of vaccination
Vaccination is based on two key elements of adaptive (acquired) immunity: specificity and memory.When an infection occurs, the body produces cells capable of fighting it and memory cells specific to the pathogen are generated. Memory cells implement a faster and stronger response on a second encounter with an infectious agent compared with the response on first exposure to the pathogen.
Vaccines allow an individual to acquire specific immunity to an infectious agent without having to suffer an initial infection. This is important because the primary response to a natural infection is often too slow to prevent serious symptoms developing.
The aim of vaccine development is to modify a pathogen (or its toxins) so that it is harmless without loss of antigenicity. This is possible because antibodies and memory cells within the immune system recognise particular areas of antigens, known as epitopes, rather than the whole organism or toxin. As long as the body is presented with such epitopes it does not need to encounter the full pathogen for specific immunity to be generated. Generally, the closer the vaccine is to the original infectious agent the better its efficacy. Live attenuated systems tend to be more effective than inactivated ones or cellular extracts (see below for further discussion of vaccine types). However, the enhanced efficacy of live vaccines is accompanied by increased adverse effects.
Identify knowledge gaps
- How do vaccines work?
- What different types of vaccine are available?
- What is the current childhood vaccination programme?
Before reading on, think about how this article may help you to do your job better. The Royal Pharmaceutical Society’s areas of competence for pharmacists are listed in “Plan and record”, (available at: www.rpsgb.org/education). This article relates to “health promotion”.
Risks and benefits of vaccines
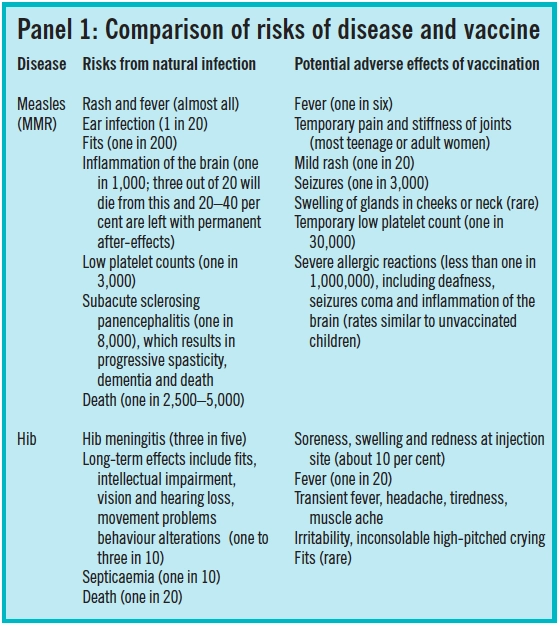
What is deemed an acceptable risk of adverse effects should be considered in relation to the risks associated with a disease. Panel 1 gives examples of the risks of vaccinations compared with their corresponding disease.
It could be argued that some anti-vaccine sentiment stems from the success of immunisation programmes. Developed countries no longer have major incidences of many diseases so people are more likely to question the need for vaccination. In developing countries, the risk versus benefit ratio can be different. For example, in developed countries, access to effective health care significantly limits mortality rates to rotavirus but in countries where access to doctors and standard fluid rehydration treatments are limited these rates soar and vaccination is more important. In 1998, Wyeth Pharmaceuticals introduced a rotavirus vaccine but, less than a year later, this was withdrawn as a result of the associated risk of intussusception which appeared in one in 12,000 children vaccinated. New rotavirus vaccines are now in clinical trials.
Types of vaccine
The type of antigen used in a vaccine depends on many factors and influences their efficacy. There are three main types of antigenic preparations currently available: live attenuated vaccines, inactivated vaccines and vaccines containing cellular extracts or toxoids.
Live attenuated vaccines
Attenuation (weakening) of live infectious agents is used to eliminate or reduce the virulence of the agent while aiming to retain its antigenicity. This can be achieved by various means, including treatment with heat, chemicals or enzymes, or genetic modification. For example, the Bacillus Calmette-Guérin (BCG) vaccine contains a live attenuated mutant of Mycobacterium bovis isolated from serial subcultures. The primary mechanism of weakening the bacillus is to remove the secreted lytic function that it needs to invade lung interstitial tissue.
Live vaccines should not be given to people with impaired immune response. For example, vaccinations with such products should be postposted to until at least three months after stopping corticosteroids or other immunosuppressants.
Inactivated vaccines
Inactivated vaccines contain whole bacteria or viruses that have been killed, usually by heat or chemicals. While generally less effective than live attenuated vaccines, inactivated vaccines offer advantages in terms of reduced adverse effects. For example, there is no risk of induction of disease via reversion or mutation to an infectious agent, as is the possibility with live attenuated systems. In 2004, within UK vaccination programmes the live polio vaccine was replaced with an inactivated one (see Panel 3 below).
In 2005, a new cholera vaccine (Dukoral) was launched in the UK. It contains killed whole Vibrio cholerae 01 bacteria. The vaccine triggers a local intestinal protective immune response, which prevents bacterium from attaching to the intestinal wall, blocking colonisation. Dukoral is an oral suspension and is added to a mixture of sodium hydrogen carbonate buffer dissolved in water.
Cellular extracts or toxoids
Vaccines based on cellular extracts (highly purified proteins, synthetic peptides, parts of cells, surface antigens) or toxoids (exotoxins detoxified, for example, by using formalin) cause fewer adverse effects than live attenuated and inactivated vaccines. Genetic engineering facilitated the construction and production of antigens that can be used. For example, the hepatitis B vaccine is cloned in yeast and manufactured using biotechnology rather than, as previously, being purified from the blood of hepatitis B carriers. This has enhanced production and safety of the vaccine and has reduced costs.
The disadvantage is that vaccines based on cellular extracts or toxoids are often poorly immunogenic and require adjuvants (substances that non-specifically enhance the immune response to an antigen.) Currently licensed adjuvants include aluminum hydroxide or phosphate (used in diphtheria, tetanus and hepatitus B vaccines) and AS04 (this contains aluminium and the bacterial lipid, monophosporyl lipid A). Toxoids, in addition to being successful vaccines in their own right (eg, tetanus), are used to increase the immunogenicity of vaccines, such as Haemophilus influenzae type b (Hib), which contains a polysaccharide unit from the bacterium conjugated to diphtheria or tetanus toxoids. New adjuvant systems being tested include particulate delivery systems, such as liposomes, niosomes, virus-based particles and microspheres.
Vaccination programmes
The goal of any vaccination is the induction of an appropriate and effective immune response, but precisely what constitutes an effective immune response for many diseases is still unclear. Efficacy is known to depend on a range of factors including age and immune status.Vaccine efficacies can range from 70 to 98 per cent effective (ie, vaccination does not guarantee immunity and not all the recipients will produce appropriate antibody levels for protection against infection).
The presence of antibodies are often considered as markers of protection. Specific levels of antibodies are considered protective (>10mIU/ml), diphtheria (>100mIU/ml) and tetanus (>100mIU/ml) but for many others, correlations of efficacy are less obvious and require further investigations.3
The length of protection conferred by a vaccine varies. For example, immunity given by hepatitis A vaccine has been estimated at between 10 and 20 years for different brands. Information can be found in the summary of product characteristics. One study of hepatitis B vaccine suggested that giving a full primary course of the vaccine to a healthy person results in lifelong immunity. However, many health care workers are advised to have boosters every five years.
The vaccines most commonly encountered by pharmacists are those for children or travellers. Panel 2 lists these.

* A new routine vaccination schedule (to take effect later this year or in 2007) is as follows:
2 months old DTap/IPV/Hib + pneumococcal vaccine
3 months old DTap/IPV/Hib + meningoccal group C vaccine
4 months old DTap/IPV/Hib + pneumococcal vaccine +meningoccal group C vaccine
12 months old Hib booster + meningoccal group C booster
13 months old MMR + pneumococcal booster
Panel 3 describes recent changes to the childhood vaccination programme.
Travellers
The vaccinations travellers require will depend on the country to be visited and the type of trip (eg, business travel or a package holiday may not present the same risks as long-term travel in rural areas) and individual circumstances need to be assessed. Travellers should be referred to their GP or a vaccination centre, but pharmacists could use queries about vaccinations as an opportunity to give advice on preventing malaria and diarrhoea.
Travellers should be advised to organise vaccinations well in advance of travel. Time is needed for courses of vaccinations to be completed (if a primary course of vaccines is required this can take up to three months), antibodies to be produced and any adverse reaction to be dealt with. For example, it can take up to four weeks for full immunity to develop following vaccination against Japanese encephalitis.
In addition, there are recommended intervals between doses and between different vaccines. It is recommended that live vaccines should be given at least three weeks apart, but the rationale behind this comes from observations that the take rates of smallpox vaccines were reduced if another live vaccine had been given shortly before. According to the Department of Health, there is no data to support this with current vaccines and, in practice, many vaccines are given simultaneously. Most inactivated vaccines can be given at the same time as other vaccines (different administration sites are used) but concurrent administration can make it difficult to determine what has caused an adverse reaction. Most courses of travel vaccines can be administered over four weeks. Some countries may require travellers to have certificates of vaccination.
Tetanus
Patients who have a wound may ask if a “tetanus jab” is needed. This depends on whether or not the patient is fully immunised (five doses of tetanus vaccine is thought to confer life-long immunity for people who do not travel outside the UK) and on the wound type. In the UK, children are given three doses of the tetanus vaccine as part of a combined vaccine (see Panel 2). This confers primary immunity and two booster doses are recommended (the first given before starting school and the second before leaving school). Patients who are not fully immunised (eg, incomplete primary immunisation or missed boosters) may require a reinforcing dose. Patients with tetanus-prone wounds (eg, puncture wounds, particularly those contaminated with soil) are likely to require a dose of tetanus immunoglobulin (to confer immediate protection) and a course of antibiotics.
Travellers are advised to have booster doses of tetanus every 10 years.
Future vaccines
Two new vaccines against the sexually transmitted human papillomavirus (HPV) are now approaching market. Some strains of human papillomavirus (eg, HPV 16 and 18) cause cervical cancer while others (eg, HPV 6 and 11) can cause genital warts. Currently, clinical trial data for both vaccines look promising with each preventing persistent infection in 100 per cent of vaccinated women and reducing cervical abnormalities by more than 90 per cent.4 The vaccines, made by Merck & Co (Gardasil) and GlaxoSmithKline (Cervarox), contain a viral protein called L1, which forms the bulk of HPV’s outer shell. Both vaccines use L1 from the HPV 16 and 18 genotype. Gardasil, however, also contains the two genotypes that cause genital warts to encourage men to be vaccinated — it is hoped that this will reduce viral spread. Both vaccines include adjuvants. Gardasil uses aluminium and Cervarox is formulated with AS04.
In anticipation of their launches ethical debate has already started to address who should get the vaccine first and how long it will take for these vaccines to be available in developing countries, which account for 80 per cent of the deaths from cervical cancer. Both Merck and GSK say they will offer the vaccine at a discount to these countries.
There remains an urgent need for new vaccines, with protection against human immunodeficiency virus and malaria being high on the wish-list. A better vaccine against TB in adults is also desirable — although the current BCG vaccine protects young children against miliary TB, it is thought to be ineffective in preventing pulmonary TB in adults. New cancer vaccine therapies, which may act by a variety of underlying mechanisms, are continuing to inch forward. In the meantime, ensuring effective participation in current vaccine programmes will have a significant impact on the control of disease and thus remains an objective for all health care professionals.
References
- Fitzpatrick M. MMR: risk, choice, chance. British Medical Bulletin 2004;69:143–53.
- Dukoral, a new cholera vaccine, licensed in the UK. Pharmaceutical Journal 2004;272:663.
- Bramwell VW, Perrie Y. The rational design of vaccines. Drug Discovery Today 2005;10:1527–34.
- Cohen J. High hopes and dilemmas for a cervical cancer vaccine. Science 2005;308:618–21.
Resources
- www.immunisation.org.uk is a good site to recommend to patients for basic information on vaccines.
- The National Travel Health Network and Centre www.nathnac.org provides health information for travellers.
- Department of Health. Immunisation against infectious disease 1996 — “the green book”. Available at www.dh.gov.uk (accessed 13 February 2006).
- Department of Health. Health information for overseas travel — “the yellow book”. Available at www.dh.gov.uk (accessed 13 February 2006).
- A CPD article to be published in September will cover influenza, including influenza vaccines.
Action: practice points
Reading is only one way to undertake CPD and the Society will expect to see various approaches in a pharmacist’s CPD portfolio.
- Write bullet points for how you would respond to a parent worried about the combined MMR vaccine; www.mmrthefacts.nhs.uk aims to provide parents and guardians with clear information on MMR.
- Evaluate how you are supporting the uptake and implementation of vaccine programmes in your area and identify how you can extend your role in offering advice on the various vaccines available for children and travellers.
- Consider the potential benefits of the new cervical cancer vaccines. Discuss with another pharmacist the ethical issues that may be associated with who (eg, adolescent girls, women, men) should get these vaccines first.
Evaluate
For your work to be presented as CPD, you need to evaluate your reading and any other activities. Answer the following questions: What have you learnt? How has it added value to your practice? (Have you applied this learning or had any feedback?) What will you do now and how will this be achieved?


