JH Naismith et al
Many bacteria wrap themselves in a sugar coating to ward off attacks from our immune systems. An international team of researchers has now unveiled a snapshot of the molecular machinery that builds these protective polysaccharide chains, giving the first clear view of a sweet spot that could be a target for new antibiotics.
It also proves that the proteins involved in creating the sugary shield rely on a molecular ruler to ensure that the polysaccharides are just the right length to offer a robust defence, according to the research published in Nature Structural and Molecular Biology
[1]
on 15 December 2014.
“Everybody knows that these rulers exist – there are lots of examples – but we’ve never seen one at a molecular level,” says James Naismith, of the University of St Andrews, Fife, who was part of the research team.
Bacteria anchor their finished polysaccharides to a lipid in their outer membrane, forming a barrier that is also a major cause of septic shock. There are more than 180 known forms of these polysaccharides, of various lengths, but each bacterium seems to make their own to a carefully regulated size. Naismith and his colleagues used X-ray and ultraviolet analysis techniques to paint a detailed picture of the polysaccharide production line in a strain of Escherichia coli.
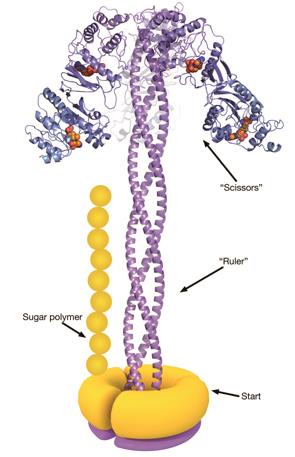
The process starts with a protein called WbdA, which is stuck to the inside of the bacterial membrane and links together mannose molecules into a polysaccharide chain. WbdA is joined to three long, helical proteins that twist together into a rigid rope – known as a coiled coil – which acts as the molecular ruler. The coiled coil is capped at the other end by another protein, WbdD[2]
, which halts the growth process when the growing polysaccharide reaches the end of the ruler.
“This is significant,” says Peng George Wang of Georgia State University in Atlanta, Georgia, who studies polysaccharide biochemistry, and was not involved in this research. “It’s been one of the big mysteries in biology for many years.”
The polysaccharides constructed by this strain of bacteria usually contain 14 mannose units, and are roughly 21 nanometres long. The researchers found that the coiled-coil ruler was 20 nanometres – perfect for building chains with the right number of sugar molecules. They also produced mutated E. coli bacteria that had smaller or larger rulers, and found that the microbes made polysaccharides that were correspondingly shorter or longer.
This is one of three processes that various types of bacteria use to make polysaccharides, and the first to be described in such detail. However, many microbes probably use the same molecular ruler, so understanding the process could eventually deliver a new way to tackle bacterial infections, says Naismith. Disrupting the function of the WbdD cap on the ruler, for example, could cause the polysaccharide to grow unchecked, rendering the molecules useless in a protective shield. In the war on infection, “we need as many targets as possible”, he says.
Wang points out that one of the major pharmaceutical uses for polysaccharides is in vaccines. The pneumococcal polysaccharide vaccine, for example, protects against infections caused by the bacterium Streptococcus pneumonia, and its efficacy depends on the length of the polysaccharide chains. “It’s an ID card for that strain of bacteria,” explains Wang.
In principle, microbes could be genetically engineered to make the desired length of polysaccharides for different vaccines, simply by changing the length of the coiled-coil molecular ruler. “I’m sure it could have practical applications,” he says.
References
[1] Hagelueken G, Clarke BR, Huang H et al. A coiled-coil domain acts as a molecular ruler to regulate O-antigen chain length in lipopolysaccharide. Nature Structural & Molecular Biology 2014. doi:10.1038/nsmb.2935.
[2] Hagelueken G, Huang H, Clarke BR et al. Structure of WbdD: a bifunctional kinase and methyltransferase that regulates the chain length of the O antigen in Escherichia coli O9a. Molecular Microbiology 2012;86:730–742.