Wikimedia Commons
An experimental single-dose influenza drug has demonstrated mixed results in treating adult patients with flu when administered within 48 hours of the onset of symptoms, according to data due to be presented at the American Society of Microbiology’s annual ICAAC meeting in Washington, DC on 8 September 2014[1]
.
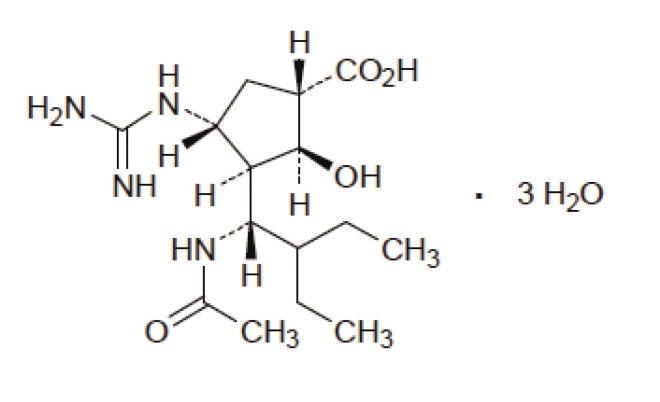
The drug, peramivir, developed by BioCryst Pharmaceuticals, failed to show significant symptom reduction when the data were adjusted for confounders but it reduced the time to resolution of fever by 24 hours, as well as reducing the amount of viral shedding in patients over the first two days.
In a separate poster presentation on 6 September 2014, peramivir demonstrated the same bioavailability whether given intravenously or by intra-muscular (IM) injection[2]
.
Source: Rich Whitley
The neuraminidase inhibitor peramivir is approved for use in Korea and Japan, where around one million patients have used the drug since 2010
Peramivir would be the first-ever single-dose influenza therapy approved in the United States if the Food and Drug Administration (FDA) endorses the application, according to William Sheridan, chief medical officer for BioCryst Pharmaceuticals in Durham, NC, who presented the poster entitled ‘Single dose injections of IV and IM peramivir are bioequivalent and well tolerated’.
The company is using the bioequivalency study to argue that the company’s research on the drug in its IM form supports FDA approval for its intravenous (IV) use, at a 600mg dose in adults with uncomplicated flu. The FDA’s decision is expected by December 2014, according to Sheridan.
Bioequivalence studies
The bioequivalence research described in the poster took place in a small number of healthy patients without flu, said Sheridan, who noted that the unblinded cross-over studies allowed subjects to serve as their own controls in comparing several doses of the drug in two formulations, IV and IM.
In the first study, 27 patients were randomised to receive 75mg, 150mg or 300mg of the drug by both IV and IM administration, with a 72 hour follow-up. The second study in the poster described the administration of 600mg of the drug in 24 patients, who also received both the IM and IV form.
Although both forms of the drug showed substantially equivalent blood concentrations, the IM injection method was associated with an increase in blood creatine kinase (CK) secretion, according to the poster.
“CK is an enzyme in every muscle cell in your body, including your heart, and if you give somebody an IM shot, it causes a tiny bit of damage to the muscle, so some of that enzyme leaks out into blood,” Sheridan explained.
In addition, the IM form of administration was painful, so the company has designed the FDA submission for IV use. “It hurts a fair bit to get the intramuscular injection, and if a patient stands up following the shot and feels faint, that could be a bad thing,” he added.
However, the IM placebo-controlled trials failed to show efficacy in an adjusted population.
“We need a drug with a different mechanism[4]
of action from existing influenza medications because we are always concerned about the development of resistance, and different drugs might act synergistically with what’s currently available,” explained Rich Whitley, of the University of Alabama at Birmingham, who is due to present the placebo-controlled IM data.
Nonetheless, peramivir, which is a neuraminidase inhibitor like existing drugs, and shows no evidence of synergy, may still be approved because of substantial scientific and clinical experience with the drug. It has been approved in Korea and Japan, where around one million patients have used the drug since[3]
2010, the company said. In addition, peramivir was also used under “emergency use authorization” regulations in the United States during the 2009-2010 global flu pandemic, in which some 2,000 patients, including children and some pregnant women, received the drug, Sheridan said.
In the IM study to be presented on 8 September 2014, ‘Single dose peramivir for the treatment of acute seasonal influenza: integrated analysis of efficacy and safety from two placebo-controlled trials’, 427 adults were randomised between 2007 and 2008 to receive either placebo or IM peramivir within 48 hours of the onset of flu-like symptoms, and were followed for 14 days.
The time taken for symptoms to resolve was slightly shorter at 113.2 hours for patients who received 300mg of the drug via the IM route than it was for those on placebo, who saw a resolution of symptoms within 134.8 hours. But although the figure had statistical significance (P=0.047), when the data were adjusted for confounders such as smoking, seasonal influenza and virus subtype, the P value was 0.161, which was not significant, the abstract says.
However, median time to resolution of fever was reduced by 24 hours, and viral shedding was significantly decreased (P=0.009), according to the abstract.
“Peramivir decreases infectivity for the first 72 hours, but I think it’s less effective in people who smoke because they have chronic underlying lung disease,” Whitley suggested.
Niche applications?
Although the company as yet lacks evidence that the agent is effective in immunocompromised patients or those with emergency conditions, there have been suggestions that peramivir may be a niche product.
“This is an IV product and, in order to get it, you would need to go to an emergency room (ER) or an urgent care clinic, or the doctor’s office, and get an infusion over 15 to 30 minutes. But if somebody is vomiting, they’re not going to keep down an oral drug, so under those circumstances it may be attractive,” noted Sheridan.
And although peramivir has not yet been tested in the UK, an ongoing controversy over studies done in the 1990s to gain regulatory approval for existing agents might make the single-dose agent a treatment of choice.
BioCryst Pharmaceuticals is not yet in talks with European regulators but is planning additional studies. “Our next study will be in children with the flu,” said Sheridan. The company hopes to start the trial within months, but does not expect it to be completed in 2014. The product is already used in children in Japan, where the dose is adjusted based on the child’s body weight, he explained.
References
[1] Whitley R, Laughlin A, Carson S et al. Single dose peramivir for the treatment of acute seasonal influenza: integrated analysis of efficacy and safety from two placebo-controlled trials. [Poster V-1297] American Society for Microbiology. Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington DC. 8 September 2014.
[2] Atiee G, Collis P, McCullough A et al. Single dose injections of IV and IM peramivir are bioequivalent and well tolerated. [Poster A-012] American Society for Microbiology. Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington DC. 6 September 2014.