Vaccinationist / Wikimedia Commons
A new experimental antifungal treatment is no less effective than its predecessor voriconazole but exposes patients to fewer side effects, a study has found.
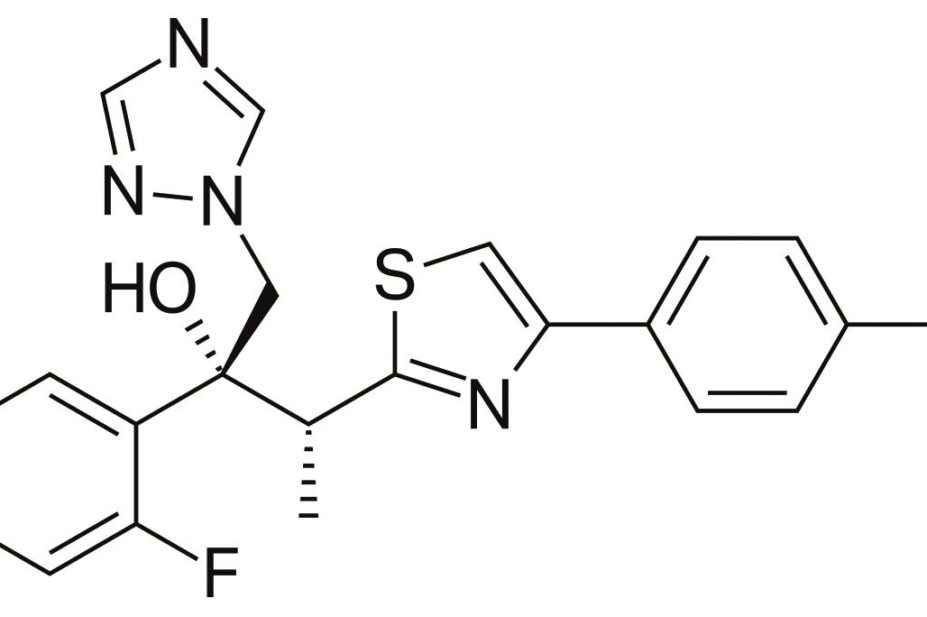
Isavuconazole can be used to treat severe fungal infection such as aspergillosis in patients with leukaemia, HIV and other immunosuppressive conditions as well as diabetes, Andrew Ullman, head of infectious diseases at the University of Wurzburg, told the American Society for Microbiology’s annual ICAAC meeting on 8 September 2014.
The broad-spectrum antifungal is being developed for the treatment of severe invasive and life-threatening fungal infections, including mucormycosis. Its developers, Astellas and Basilea Pharmaceutical International are seeking market approval from the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Decisions from both agencies are expected in 2015.
Ullman, along with Kieren Marr, professor of medicine at Johns Hopkins School of Medicine in Baltimore, discussed isavuconazole before a poster presentation[1]
about the drug. Ullman said serious fungal infections were on the rise due to increasing numbers of immunosuppressed patients.
The rate of fungal infection without mycological evidence in leukaemia patients is between 15% and 20%, he said, and even higher in patients with uncontrolled cancer.
“The disease is not that frequent, but its epidemiology is deadly,” said Ullman. All-cause mortality by day 42 in the study for those patients with uncontrolled cancer (out of a total study population of 516) was 21% for those on isavuconazole, compared with 22% for those treated with voriconazole, the predecessor drug in the same class. But voriconazole was associated with side effects, including kidney toxicity, heightened risk for skin cancer and hallucinations, according to Marr. That said, those without uncontrolled cancer showed a lower overall response if they took the experimental drug. Of these patients, 43% (17 patients) who received voriconzaole had an overall response, compared with 33% (18 subjects) who took isavuconazole (difference 9.2, 95% confidence interval −13.1 to 31.4).
A separate poster[2]
, presenting data from a subset of patients from the same trial, also revealed a better side effect profile for isavuconazole compared with voriconazole. In that trial, 516 patients were enrolled between 2007 and 2013 in Asia, Australia, Europe, Latin America and North America and randomised to either anti-fungal drug. Any patient who received at least one dose of isavuconazole was included in the intent-to-treat population, and all-cause mortality was assessed after 42 days.
Of those patients with uncontrolled cancer, 40% (70 subjects) who received isavuconazole experienced drug-related adverse events, compared with 60% (112 people) of those who received the already-licensed drug (P<0.05).
Of those with uncontrolled cancer who received voriconazole for their fungal infections, 24% (44 subjects) experienced problems related to the eye, compared with 15% (26 individuals) who were randomised to receive the experimental agent.
Of the 516 patients, 272 had uncontrolled malignancy, while others had cancer that was in remission, rheumatoid arthritis or HIV. Some had diabetes, a substantial number of whom had mucormycosis, Marr said.
For those subjects without an uncontrolled malignancy, 35% (25 individuals) of those on voriconazole had eye-related side effects, compared with 15% (13 patients) of those who received isavuconazole, the poster said.
The experimental drug was also associated with fewer cases of liver toxicity in patients without uncontrolled cancer. Four subjects (5%) without uncontrolled malignancy who received isavuconazole experienced liver problems compared with 14 subjects (19%) who were randomized to voriconazole.
“The drug had 100% bioavailability,” noted Ullman, suggesting that this should ease concerns about unpredictable and inconsistent blood levels with a predecessor drug.
References
[1] Ullmann AJ, Shoham S, Huang W et al. A phase 3 randomized, double-blind, non-inferiority trial evaluating isavuconazole (ISA) vs voriconazole (VRC) for the primary treatment of invasive fungal disease (IFD) caused by Aspergillus spp. or other filamentous fungi (SECURE): outcomes by malignancy status. [Poster M-1756] American Society for Microbiology. Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington DC. 8 September 2014.
[2] K. Marr, E. Bow, W. Heinz et al. A phase 3 randomized, double-blind, non-inferiority trial evaluating isavuconazole (ISA) vs voriconazole (VRC) for the primary treatment of invasive mold infection (SECURE): Outcomes in subset of patients with hematologic malignancies (HM). [Poster M-1757] American Society for Microbiology. Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington DC. 8 September 2014.