CID - ISM / Science Photo Library
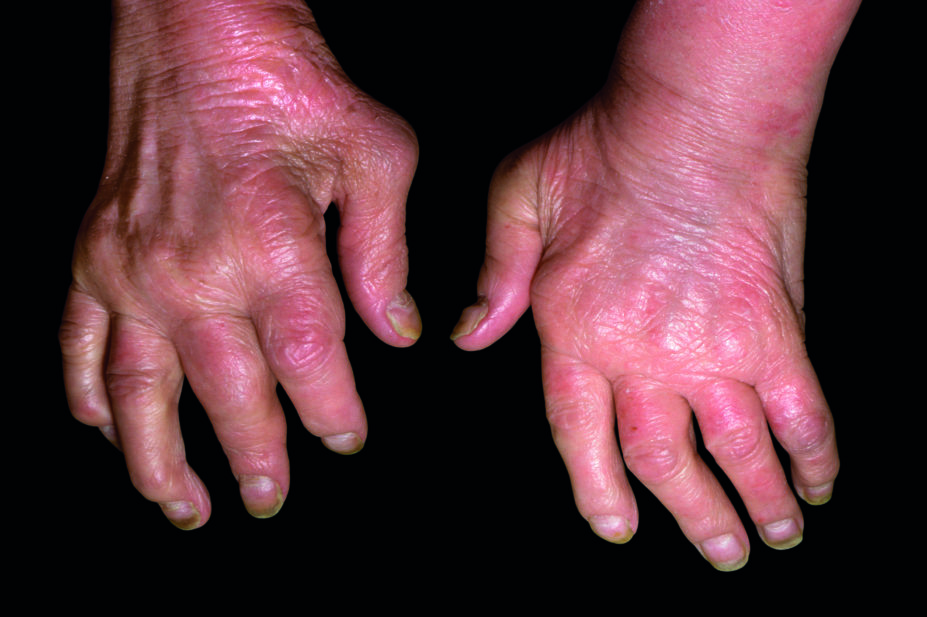
There are limited treatment options for people with psoriatic arthritis who either do not respond to or are unable to take tumour necrosis factor (TNF) inhibitors.
In a phase III trial, published in The Lancet
[1]
(online, 24 May 2017), 363 people with refractory psoriatic arthritis were randomly assigned to placebo or ixekizumab, a monoclonal antibody that targets interleukin 17A, administered every two or four weeks.
After 24 weeks, more people who received ixekizumab every four weeks had at least a 20% significant improvement in the American College of Rheumatology response criteria compared with those who received placebo (53% vs 20%). The same was also true of people who received ixekizumab every two weeks (48%).
The researchers conclude that ixekizumab, which has already been shown to be effective in treatment-naive patients, is an alternative option for difficult-to-treat psoriatic arthritis patients who have had an inadequate response, or intolerance, to TNF inhibitors.
References
[1] Nash P, Kirkham B, Okada M et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. Lancet 2017; doi: 10.1016/S0140-6736(17)31429-0