MHRA/RPS
The Royal Pharmaceutical Society has worked with the Medicines and Healthcare products Regulatory Agency (MHRA) and the Royal College of General Practitioners to produce a short video about the risks of valproate, a medicine prescribed for epilepsy and bipolar disorder. The video is aimed at GPs, pharmacists and other healthcare professionals and gives an overview of the latest MHRA guidance on discussing the medication with patients.
It follows an announcement by the European Medicines Agency (EMA) that it will host a public hearing during which patients can share their experiences of taking valproate. The hearing, which takes place at the EMA’s London office, forms part of a review by the EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) on the risks and benefits of valproate to women and girls of childbearing age.
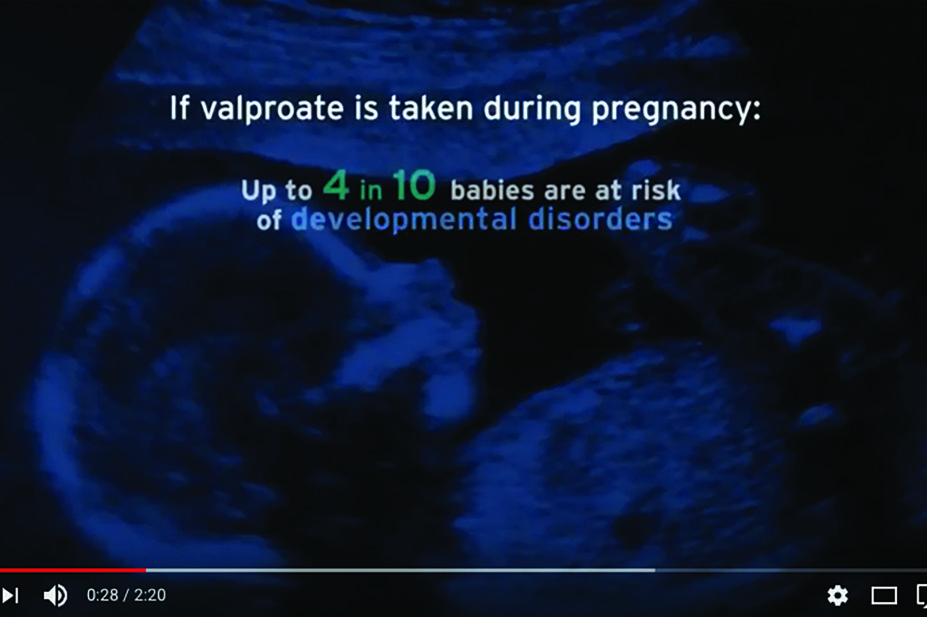
Valproate is associated with serious harm to foetal development. It can cause birth defects and developmental disorders, and should not be taken by women and girls unless no other medication is effective. In April 2017, the RPS released a Quick Reference Guide to dispensing valproate for girls and women.
The MHRA said all women and girls taking valproate should be informed of the risks as well as the benefits of the medication.
The new video accompanies a toolkit on the risks of valproate previously developed by the three health bodies. Both the video and the toolkit may be accessed on the MHRA website. The video is also available on YouTube.
You may also be interested in
Long service of members

Membership fees 2022
