Saturn Stills / Science Photo Library
Summary
The management of asthma in the UK is based on guidance from the British Thoracic Society and Scottish Intercollegiate Guidelines Network (BTS/SIGN), which uses a stepwise approach. Patients with asthma should have a personalised asthma action plan (PAAP) and be given advice on self-management and inhaler technique.
Inhaled short-acting beta-2 agonists are used at step 1 of treatment. Inhaled corticosteroids are the most effective anti-inflammatory medicines used for symptomatic and persistent asthma and should be first introduced at step 2 of asthma management. Further steps include adding a long-acting beta-2 agonist, adding a fourth drug such as a leukotriene receptor antagonist or theophylline, and using a daily corticosteroid tablet.
Guidance on the management of asthma is produced by the British Thoracic Society and Scottish Intercollegiate Guidelines Network (BTS/SIGN) and was updated in October 2014[1]
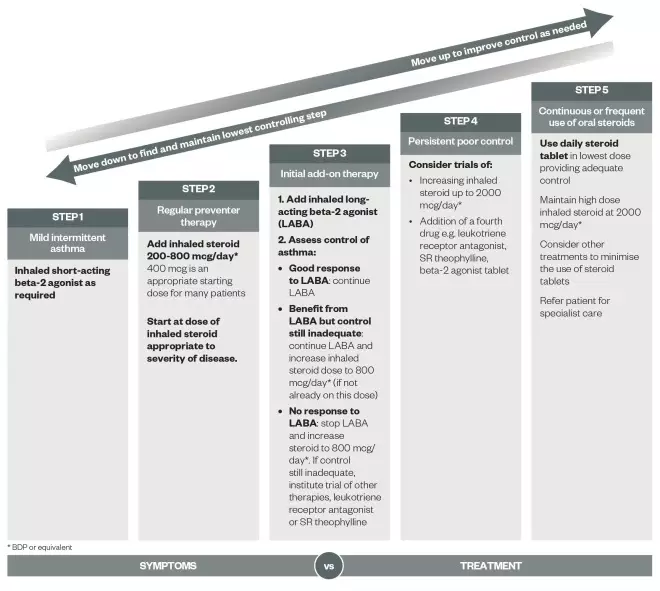
. The guidelines advocate a stepwise approach to asthma management (See ‘Stepwise treatment of asthma in adults’).
An asthma attack is a medical emergency and requires immediate treatment. The emergency management of an asthma attack is outside the scope of this article.
Source: BMJ Publishing Group Ltd
Stepwise treatment of asthma in adults
Patients should start treatment at the step most appropriate to the initial severity of their asthma. Check concordance and reconsider diagnosis if response to treatment is unexpectedly poor.
Pharmacological treatment
Inhaled short-acting beta-2 agonists (SABAs)provide short-term relief for mild and intermittent asthma and are prescribed when required as step 1 treatment and throughout the BTS/SIGN asthma steps. SABAs act on beta-2 adrenoceptors on bronchial muscle causing bronchodilation and temporary relief of symptoms. Examples include salbutamol and terbutaline. Patients using high doses of SABAs are more likely to suffer adverse effects, such as tremor, cramps, palpitations and headache.
Patients increasing their use of a SABA is a marker of uncontrolled asthma and indicates that therapy should be escalated to the next step. BTS/SIGN step 2 asthma treatment is indicated for patients who:
- Have had an exacerbation of asthma in the preceding two years;
- Experience asthma symptoms three times a week or more;
- Are woken up at night with asthma symptoms on one or more occasion per week.
Asthma is an inflammatory disease and the threshold for stepping-up therapy to include prophylaxis treatment is set deliberately low to ensure the underlying pathology of asthma is managed. Before a step-up in therapy is initiated (at each step), adherence to inhaled therapy and inhaler technique should be checked and optimised where appropriate.
Inhaled corticosteroids (ICSs) are the most effective anti-inflammatory medicines used for symptomatic and persistent asthma and should be first introduced at step 2 of asthma management. ICSs reduce inflammation in the airways and airway hyperresponsiveness, and, with regular use, can reduce the severity and frequency of exacerbations, improve quality of life and lung function and reduce mortality[2]
.
The recommended starting dose for adults is 400µg of beclometasone dipropionate equivalence per day (see ‘Beclomethasone dipropionate equivalence ratio of inhaled corticosteroids’). Treatment should be adjusted as necessary to the lowest effective dose that achieves asthma control.
| Beclomethasone dipropionate equivalence ratio of inhaled corticosteroids[1],[3],[4] | |
|---|---|
Drug | BDP Equivalence Ratio |
Beclometasone dipropionate – CFC | 1:1 |
Beclometasone dipropionate – QVAR | 2:1 |
Budesonide | 1:1 |
Ciclesonide* | 2.5:1 |
Fluticasone propionate | 2:1 |
Fluticasone furoate** | 10:1*** |
* Ciclesonide is a newer medicine and its dose equivalence and safety profiles are not well established ** 92µg equivalent to fluticasone propionate 500µg, which is equivalent to 1,000µg of beclometasone dipropionate *** This is current practice, although it could be reduced to 5:1 in the future | |
Current and previous smoking reduces the effect of ICSs; therefore, higher doses may be required to achieve control.
There have been concerns raised over long-term side effects of ICS therapy, such as diabetes, skin thinning and bruising, and cataracts. However, there is little evidence that doses below 800mcg beclometasone dipropionate per day cause any detrimental effects apart from the local side effects[1]
. Higher doses of ICSs have the potential to induce adrenal suppression so patients should be given a “steroid card”. Such patients may also need corticosteroid cover during an episode of stress (for example, an operation).
Local side effects, such as dysphonia and oral candidiasis, are more common with ICS therapy. These local effects may be minimised by using a spacer device with pressurised metered dose inhalers and rinsing the mouth after each use. Doses of 800µg beclometasone dipropionate per day or equivalent are associated with greater occurrence of side effects.
ICSs are a highly effective way of treating asthma, and in most people the benefit of this effective treatment is much greater than any risk of side effects.
Discontinuation of treatment can worsen clinical outcomes quickly (within weeks or months)[2]
. The National Institute for Health and Care Excellence (NICE) has developed guidance to support practitioners in stepping patients’ treatment down when needed and specifically identifying when high doses of ICSs should be reviewed. It advises that patients who are on an equivalent of 800µg of beclometasone dipropionate or higher and have been stable for over three months should have their dose reviewed and reduced by 25–50% if clinically appropriate[5]
[6]
.
Long-acting beta-2 agonists (LABAs) should be the first choice of add-on therapy for patients who continue to have uncontrolled asthma on ICS therapy. Many patients benefit more from adding a LABA to therapy at step 3 than from increasing the dose of ICS. (LABAs should be considered before increasing doses of ICSs above 400µg beclometasone dipropionate or equivalent per day and must be considered before using doses greater than 800µg.)
LABAs produce a longer duration of bronchodilation compared with short-acting beta-2 agonists. However, they should not be used as a single therapy for asthma treatment and a US Food and Drug Administration meta-analysis found LABAs without simultaneous use of ICSs was associated with increased asthma morbidity and possibly mortality[7]
.
Adding a LABA to ICS therapy has been shown to improve lung function and asthma symptoms, and decrease exacerbations. LABAs do not affect airways inflammation and should not be used alone for the treatment of asthma. They should always be prescribed in combination with an ICS to aid adherence. Adverse effects associated with LABAs include cardiovascular stimulation, anxiety and tremor.
If adding in the first choice LABA is not effective, second-choice therapies, such as leukotriene receptor antagonists (LTRAs), theophylline, oral beta-2 agonists or long-acting muscarinic antagonists (LAMAs), can be considered.
Leukotriene receptor antagonists (LTRAs), such as montelukast and zafirlukast, may provide improvements in lung function, reduce exacerbations and improve asthma symptoms. They interfere with the pathway of leukotriene mediators, which are released from mast cells, eosinophils, and basophils. LTRAs are an alternative therapy for the treatment of patients who require step 3 care.
The advantages of LTRAs are that they are orally administered and have few adverse effects. LTRAs may be of particular benefit in patients with exercise-induced asthma or patients who are aspirin-intolerant or have concomitant rhinitis.
Theophylline promotes bronchial smooth muscle relaxation, increases mucociliary transport and contractility of the diaphragm, and acts as a central respiratory stimulant. In clinical practice, theophylline improves lung function and symptoms but side effects are more common than with alternative treatments. Possible side effects include tachycardia, palpitations, headache, insomnia, nausea and other gastrointestinal disturbances.
Theophylline is less effective than LABAs in the treatment of asthma but can be a useful addition to a patient’s treatment when control is suboptimal. It is important to adjust the dose of theophylline prescribed to ensure that a plasma level is achieved for satisfactory bronchodilation; in most patients this is 10–20mg/l. Adverse effects can occur within this range, and their frequency and severity increase in plasma levels above 20mg/l.
Oral beta-2 agonists, given as slow-release tablets, may improve lung function and symptoms. However, these are rarely used in practice.
Long-acting muscarinic antagonists (LAMAs) appear to be as effective as salmeterol in the short-term control of asthma, and may be more effective than doubling the dose of ICSs in fixed airways obstruction. Currently, the only LAMA licensed for asthma is tiotropium (via the Respimat device). Longer-term studies are required to confirm the place of LAMAs in the treatment of asthma.
Maintenance and reliever therapy is an approach to therapy that may be suitable for some patients at step 3 and above of the BTS/SIGN guidelines who struggle with using multiple inhalers. It uses combination products that contain a LABA and ICS to provide preventive maintenance and reliever therapy without the need for any additional reliever therapy with a SABA. The products Symbicort (budesonide and formoterol) and Fostair (beclometasone and formoterol) are the only two inhalers that are licensed to be used in this way.
Patients receive a maintenance dose of ICS/LABA in the morning and at night, and then as needed for relief of their symptoms. When using this approach, the total regular dose of ICSs should not be decreased from their usual maintenance dose. Furthermore, patients who use the inhaler as a reliever one or more times during the day on a regular basis, in addition to their maintenance dose, should have their treatment plan reviewed[1]
. If using this approach, careful patient education is essential to ensure appropriate use[1],[8]
.
Oral corticosteroid maintenance therapy is required for a small proportion of patients who have severely uncontrolled asthma (step 5 of the BTS/SIGN guideline). Prednisolone is most commonly prescribed corticosteroid for this indication[1]
. Efforts should be made to minimise systemic adverse effects, such as using the lowest dose possible that controls symptoms. Long-term side effects of oral corticosteroids include hypertension, diabetes mellitus, hyperlipidaemia and osteoporosis. Patients who require long-term courses lasting more than three months and those who require frequent courses (three or more in one year) should be monitored closely for any changes in blood pressure, plasma or urine glucose levels, cholesterol and bone mineral density. Other adverse effects of long-term oral corticosteroids include obesity, cataracts, glaucoma, skin thinning and bruising, and muscle weakness.
Other therapies
Omalizumab is a humanised anti-immunoglobulin E (IgE) monoclonal antibody. It binds to circulating IgE, reducing the levels of IgE available to bind to mast cells and initiate the allergic cascade that often results in an exacerbation of asthma. It is licensed for adults and children over the age of six years who have allergic asthma. Omalizumab has been approved by NICE as an add-on therapy for patients who require continuous or frequent treatment with oral corticosteroids (four or more courses per year[9]
).
In England, NICE has approved the use of omalizumab through a patient access scheme provided by the manufacturer (otherwise it was not deemed cost effective)[9]
. It is administered by subcutaneous injection every two or four weeks, depending on the patient’s weight and IgE level. It has been shown to substantially reduce severe exacerbations, reduce reliever use and improve quality of life[1],[9]
. NHS England has stipulated that omalizumab may only be initiated in centres that are designated “difficult asthma centres”.
Immunosuppressants, such as methotrexate, ciclosporin and oral gold, have been shown to reduce the need for long-term oral corticosteroids. However, there is marked variability in patient response and this approach can often cause significant adverse effects[1]
. Furthermore, there is no evidence that beneficial effects continue after stopping treatment[1]
.
For patients who require frequent courses or long-term daily dosing of oral corticosteroids to control their asthma, a three-month trial of an immunosuppressant may be prescribed, providing the risks and benefits of treatment have been discussed with the patient. Careful monitoring is required and this form of therapy should only be started in patients who are under the care of specialist asthma centres which have experience of using these medicines[1]
.
Long-term continuous subcutaneous terbutaline infusions have been used for patients with severe asthma. However, the efficacy and safety of this has not been assessed through a randomised controlled trial[1]
. The use of anti-TNF alpha therapy has also been investigated in severe asthma.
Recent research has been concerned with the introduction of novel therapies for asthma such as thermal bronchoplasty (a procedure involving delivery of radiofrequency energy to the airway wall to heat the tissue and reduce smooth muscle present) or medicines that target specific components of the anti-inflammatory pathway. These include additional monoclonal antibodies for IgE, interleukin-4, interleukin-5 (mepolizumab and benralizumab) and interleukin-13 (tralokinumab).
These treatments are invasive, expensive and can be associated with a higher level of risk, and therefore are likely to be reserved for patients with difficult-to-treat asthma. The new medicines being developed offer a more personalised approach to asthma care whereby patients could undergo “biomarker” blood tests to check if they are likely to respond to the medicines.
Severe and difficult asthma
Most patients have mild disease that is readily controlled using low-to-moderate doses of ICSs, with or without a LABA. However, between 5% and 10% of asthmatics have disease that is more difficult to control and experience persistent symptoms and frequent exacerbations[10]
. Morbidity and health costs are disproportionately high for these patients and they are at greater risk of fatal and near-fatal exacerbations. In addition, the regular prescribing of oral and high-dose ICSs increases the risk of corticosteroid-related adverse effects.
Different international task forces, workshops, guideline committees and authors have used terms such as ‘severe’, ‘refractory’ and ‘difficult’ to describe the condition in this population. However, there is no universally accepted definition. The most recent consensus is to distinguish between ‘severe refractory asthma’ and ‘difficult asthma’. Patients who have persistent symptoms, frequent exacerbations, or both, despite treatment at step 4 or step 5 of the BTS/SIGN guidelines, are described as having difficult asthma[1]
. In many cases, difficult asthma is a result of poor adherence to therapy, incorrect inhaler technique, environmental factors, psychological issues or co-existing conditions.
In contrast, patients with severe refractory asthma are those patients who have difficult asthma (as described above) but remain uncontrolled despite resolution of other contributing factors. Patients require a rigorous and systematic approach to their diagnosis and treatment. Ideally, assessment should be facilitated through a dedicated multidisciplinary service by a team experienced in the assessment and management of difficult asthma.
Personalised care
Patients with asthma should be offered self-management training that focuses on individual needs. Personalised asthma action plans (PAAPs) can help patients recognise the deterioration of asthma control and provide tailored advice on treating the exacerbation in the early stages. The recognition of deteriorating asthma requires interpretation of both subjective and objective measures of asthma severity. The PAAP should inform patients about when and how to modify medicines in response to worsening asthma and how and when to see a healthcare professional in response to worsening asthma.
Each patient’s PAAP should include the following:
- Instructions on how to recognise signs of worsening asthma
- Advice on the prompt use of SABAs and oral corticosteroids
- Monitoring of response to medicines
- Contact information / telephone numbers
- Follow-up to assess asthma control
Inhaler technique
Education regarding correct inhaler technique is essential. Studies have shown that many patients are unable to use their handheld inhalers appropriately, reducing the efficacy of the medicine[11]
. Inhalers should be prescribed only after patients have received training in the use of the device and have demonstrated satisfactory technique. Technique should be checked on a frequent basis to ensure ongoing ability to use the device correctly[1]
.
References
[1] Joint British Thoracic Society/Scottish Intercollegiate Guidelines Network. British Guideline on the Management of Asthma. Clinical Guideline 141. Thorax 2014;69:suppl1.
[2] Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Online 2012. Available at: http://www.ginasthma.org (accessed 6 June 2014).
[3] National Prescribing Centre. Current issues in the drug treatment of asthma. MeReC Bulletin. 2008;19:2.
[4] GlaxoSmithKline. Relvar Ellipta 92mcg/22mcg inhalation powder. Summary of Product Characteristics. January 2014.
[5] National Institute for Health and Clinical Excellence. High dose inhaled corticosteroids (ICS) in asthma. London:NICE 2012.
[6] National Prescribing Centre. Key therapeutic topics — medicines management options for local implementation. Manchester: NPC 2012.
[7] Pesola G, Lone T & Gosala R. Long-acting beta agonists and their relation to increased asthma morbidity and mortality. The FDA meta-analysis. Internet J Asthma, Allergy and Immunol. 2008;7:1.
[8] Papi A, Corradi M, Pigeon-Francisco C et al. Beclomethasone-formoterol as maintenance and reliever treatment in patients with asthma: a double-blind, randomised controlled trial. Lancet Respiratory Medicine 2013;1:23–31.
[9] National Institute for Health and Care Excellence. Omalizumab for treating severe persistent allergic asthma. TA278. London: NICE 2013.
[10] Strek ME. Difficult asthma. Proc Am Thorac Soc 2006;3:116–123.
[11] Broedersa MEAC et al on behalf of the ADMIT Working Group. The ADMIT series — Issues in inhalation therapy. 2) Improving technique and clinical effectiveness. Prim Care Respir J 2009;18:76–82.
You might also be interested in…

Helping respiratory patients breathe easy
Asthma therapies get personal