Tic disorders are hyperkinetic movement disorders characterised by the presence of tics (involuntary, sudden, rapid, recurrent, non-rhythmic movements or vocalisations)[1]
. Tic symptoms are best viewed along a continuum; a distinction between motor tics and vocal tics does not appear to reflect separate pathophysiological processes and does not bear implications for the management of tic symptoms. In practical terms, vocal tics can be described as motor tics accompanied by sounds (caused by involvement of respiratory muscles). Moreover, the term ‘phonic tics’ could be a better descriptor than ‘vocal tics’, given that not all vocal tics are produced by the vocal chords. Tourette syndrome is the most complex and clinically relevant tic disorder: it is more appropriately referred to as Gilles de la Tourette syndrome, after Georges Gilles de la Tourette, who published the first complete description of this syndrome in 1885[2]
, following a previous report on the “tic non douloureux” by Armand Trousseau[3]
. Tourette syndrome is a neurodevelopmental condition characterised by multiple motor and vocal/phonic tics, which typically develop in early childhood and are often accompanied by co-morbid behavioural, emotional and psychological symptoms[4]
,[5]
.
Tourette syndrome is no longer considered to be a rare medical condition, as large epidemiological studies and meta-analyses have shown that 0.3–1.0% of school-age children fulfil established diagnostic criteria for the disorder[6]
. In the UK, it is estimated that as many as 200,000 to 330,000 individuals have symptoms consistent with Tourette syndrome, with varying degrees of severity[7]
. Tics as isolated symptoms are estimated to be more common, potentially affecting around 5% of the general population at some point[8]
, although prevalence figures show a wide variability, ranging from 1% to 29% depending on the different study designs, methodologies and sampled populations[9]
. For example, the incidence of tics is known to be higher in children than adults, more common in boys than girls, and more prevalent in special education settings[10]
. This article reviews current knowledge about the diagnosis and management of different types of tic disorders, with a focus on Tourette syndrome.
Pathophysiology
The exact pathophysiology and brain mechanisms associated with tic development and expression are poorly understood. Converging evidence from neuropharmacology, as well as structural and functional neuroimaging, indicates dysfunction of the dopaminergic transmission within cortico-striato-cortico-frontal circuitries as a key aetiological pathway[11]
. The few neuropathological studies of patients with tic disorders have provided preliminary evidence for a deficit in cerebral maturation, mainly at the level of striatal interneuron migration[11]
. It has also been suggested that the presence of chronic tics, and/or the continuing attempts to suppress them, can induce neuroplastic remodelling of brain structures such as the corpus callosum[12]
. Fluctuations in tic severity may also be modulated by the activity of the autonomic nervous system[13]
. Genetic predisposition has a major role in the development of Tourette syndrome, as shown by early family studies. The failure of Mendelian transmission models to account for the vast majority of familial cases of Tourette syndrome leads to the hypothesis of polygenic and bilineal transmission mechanisms. Further research has highlighted the complexity of possible heritability pathways, indicating that Tourette syndrome is a genetically heterogeneous disorder, with vulnerability loci scattered throughout the genome[14]
. As in most neuropsychiatric disorders, environmental factors have been proposed to play a contributory role. Specifically, epidemiological and laboratory studies have suggested that respiratory infections and autoimmune dysfunction, as well as prenatal and perinatal problems, can be involved in the aetiopathogenesis of at least a subgroup of patients with Tourette syndrome[15]
. The hypothesis that Tourette syndrome is among the neuropsychiatric conditions collectively referred to as Paediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infections (PANDAS) is still controversial. The concepts of genetic and aetiological heterogeneity are in line with the results of principal component factor analysis and hierarchical cluster analysis studies conducted over the past decade, which confirmed the existence of multiple phenotypes within the Tourette syndrome spectrum[16]
.
Signs and symptoms
Tic disorders manifest early in life (average age at onset is around six years; male to female ratio 4:1), usually with simple motor tics[1]
,[4]
. The most common tic-type reported at onset is eye blinking[17]
, followed by other simple tics such as eye rolling, mouth opening, facial grimacing, shoulder shrugging, neck stretching, kicking and abdominal contractions (see ‘Common motor and vocal/phonic tics’).
| Table 1: Common motor and vocal/phonic tics | ||
| Tics | Simple | Complex |
| Motor | Eye blinking, eye rolling, jaw movements, neck stretching, shoulder shrugging, abdominal crunching, arm stretching | Forced touching, abnormal gait, whole body movements, copying others’ movements (echopraxia), making rude gestures (copropraxia) |
| Vocal/phonic | Sniffing, grunting, throat clearing, coughing, snorting, squeaking, humming | Repeating own words (palilalia) or others’ words (echolalia), making obscene/offensive remarks (coprolalia) |
Complex motor tics tend to develop later in life and encompass motor patterns that involve multiple muscular segments, resulting in whole body movements or abnormal gait. In terms of vocal/phonic tics, the most frequently reported ones are sniffing, grunting, throat clearing, humming, as well as simple vocalisations (e.g. “ah” and “ooh”). In addition to these simple vocal/phonic tics, a minority of patients can present more complex symptoms (e.g. uttering entire words). Interestingly, Georges Gilles de la Tourette’s original case series described nine patients who were known to have complex vocal/phonic tics, namely echolalia (repeating other people’s words) and coprolalia (swearing as a tic)[2]
. Palilalia is a complex vocal/phonic tic that manifests itself with the repetition of the patients’ own words, usually for a set number of times or until they “sound right”. The motor equivalents of these complex tics are echopraxia (copying other people’s actions), copropraxia (involuntary production of rude gestures) and palipraxia (repetition of own actions). Despite wide media coverage received in recent years, coprolalia is relatively rare, documented in around 10% of patients with Tourette syndrome in the community (up to 30% in specialist clinics, where more severe/complex cases tend to be seen)[18]
.
Patients often report that their tics are preceded by a subjective feeling of mounting inner tension and that this is temporarily relieved by tic expression[19]
,[20]
. The presence of these “premonitory urges” to tic is taken into consideration by clinicians for the differential diagnosis between tics and other repetitive behaviours, such as stereotypies and mannerisms. While the absence of rhythmicity makes it easy to distinguish tics from tremor, some patients present with dystonic tics (slow and sustained muscle contractions) or catatonic features as part of their tic repertoire, which can pose considerable diagnostic challenges[21]
. Assessing the degree of tic suppression (at the expense of mounting inner tension and subsequent rebound in tic severity) is also helpful in differential diagnosis.
Patients with tics are aware that their symptoms tend to follow a waxing and waning course, with significant variations in frequency, severity and distribution throughout life. There is a need for longitudinal studies in patients with tic disorders, however it is known that tics usually peak in severity during early teenage years and in the majority of cases show a tendency to improve or stabilise after puberty[22]
. Of note, tics persist into adulthood in approximately 90% of cases, although severity may be attenuated. Environmental factors can influence tic expression to a considerable extent. Stress, anxiety, psychological pressure and self-consciousness, as well as excitement, have been reported as the main aggravating factors, whereas ameliorating factors include relaxation and engagement in non-stressful mental and physical tasks requiring active concentration (e.g. sports, music). Social interactions are also known to modulate tic severity[23]
, including the expression of particular socially-inappropriate behaviour[24]
.
Overall, tics can have a variable degree of severity, ranging from mild twitches that do not cause significant impairment and often go unnoticed, to forceful movements and loud noises that can sometimes cause injury and catch other people’s attention. Moreover, converging evidence shows that around 90% of patients with Tourette syndrome present with associated behavioural and/or psychological disorders[16]
,[25]
,[26]
, most commonly obsessive compulsive disorder (OCD) or behaviours (approximate comorbidity rate for both is 30%), attention deficit and hyperactivity disorder (ADHD) (approximate comorbidity rate for both is 60%), autistic spectrum disorders (ASD), affective disorders and impulse control disorders[27]
,[28]
,[29]
. The recent development of disease-specific health-related quality of life measures for patients with Tourette syndrome[30]
,[31]
,[32]
has allowed an accurate assessment of the differential impact of tics and behavioural comorbidities on patients’ well-being, taking into account symptom severity as well as psychological factors and social circumstances[33]
.
Diagnosis
The current edition of the Diagnostic and Statistical Manual for Mental Disorders (DSM-5) classifies tic disorders as movement disorders within the ‘Neurodevelopmental disorders’ section[34]
. Specifically, the DSM-5 lists four criteria for Tourette syndrome (DSM-5 code 307.23):
1. The presence of both motor and vocal/phonic tics at some time during the illness (not necessarily concurrently);
2. The persistence of tics for more than one year since first tic onset, irrespective of the duration of tic-free periods;
3. The onset age before 18 years;
4. The absence of underlying organic causes for the tics.
The impairment criterion was removed from the DSM in 2000, with the text revision of the fourth edition. However, problems in social functioning are frequently reported by patients with Tourette syndrome, and include the presence of autism traits below and above diagnostic thresholds and other social behaviours such as non-obscene socially-inappropriate behaviours[24]
. Persistent (chronic) motor or vocal tic disorder (DSM-5 code 307.22) is diagnosed when single or multiple motor or vocal/phonic tics (but not both motor and vocal/phonic tics) are present during the illness, and criteria for Tourette syndrome are not fully met. The category of provisional tic disorder (DSM-5 code 307.21) captures patients with single or multiple motor and/or vocal/phonic tics that have been present for less than one year since onset. The other specified tic disorder category (DSM-5 code 307.20) should be applied when there is strong evidence from the history, physical examination and/or laboratory results to suggest a plausible underlying cause for the tic symptoms (e.g. Huntington’s disease, post-viral encephalitis, cocaine use), when the age at tic onset is above 18 years, and in general when tics are present but full criteria for a tic/neurodevelopmental disorder are not met. Finally, the unspecified tic disorder category (DSM-5 code 307.20) can be used in situations where there is insufficient information to make a more specific diagnosis in patients with tics who do not meet the criteria for a tic/neurodevelopmental disorder. The diagnostic criteria proposed by the World Health Organization – International Classification of Diseases[35]
and by the Tourette Syndrome Classification Study Group (TSCSG)[36]
show a considerable overlap with the outlined DSM criteria, with minor differences (e.g. age at tic onset under 21 years rather than 18 years according to the TSCSG criteria).
The diagnosis of Tourette syndrome requires expert observation and in-depth history-taking to document the chronic presence of multiple motor and vocal/phonic tics since childhood. Specific investigations, including laboratory tests and neuroimaging, are only indicated in particular cases to rule out possible underlying causes for tics in patients with atypical presentations, such as acute onset, onset in adulthood, or sustained/dystonic tics. Interestingly, complex symptoms such as coprolalia and echolalia, which were originally mentioned by Georges Gilles de la Tourette as core features of the condition he described, do not feature among the current diagnostic criteria.
The high prevalence of co-morbid obsessive compulsive features and attention deficit and hyperactivity symptoms can pose considerable diagnostic challenges, even to experienced clinicians. Both complex tics and compulsions can be described as repetitive behaviours of a similar nature; however, in-depth assessment reveals specific differences that are important aspects of differential diagnosis[37]
. Unlike tics, the compulsions reported by patients with OCD are often purposeful, ritualistic and routine-like in nature. Moreover, compulsive behaviours are performed as a method to reduce psychological anxiety caused by intrusive (obsessional) thoughts, whereas tics are driven by premonitory urges that are more physical in nature and are often described as sensations of somatic discomfort, pain or tension. Interestingly, patients with tics often report the presence of urge-driven obsessive compulsive behaviours that are clinically different from the symptoms reported by patients with a primary diagnosis of OCD[38]
. For example, in patients with Tourette syndrome there is a significantly higher prevalence of concerns for symmetry, evening-up behaviours, obsessional counting (arithmomania), ordering and “just-right” perceptions, whereas patients with pure OCD have a higher rate of concerns for contamination and cleaning/washing rituals. These phenomenological differences are likely to reflect different pathophysiological mechanisms and should inform targeted management pathways. The diagnosis and management of children and adolescents with Tourette syndrome can be complicated by the co-occurrence of attention deficit and hyperactivity symptoms. By definition, children with tics are restless and hyperactive; moreover, the constant efforts they have to produce in order to actively suppress their tics can interfere with their ability to sustain concentration in school settings. It is therefore recommended that the diagnosis of co-morbid ADHD is established by experienced child and adolescent psychiatrists, paediatricians or adult psychiatrists/neuropsychiatrists after a comprehensive clinical assessment, which can involve collecting information from parents and teachers[39]
.
Treatment
Although it is estimated that around two-thirds of patients with tic disorders improve to some extent by adulthood, more severe forms of Tourette syndrome can affect patients’ health-related quality of life throughout their lives[40]
. In reality, the majority of children with Tourette syndrome will have relatively mild symptoms that require only psychoeducation so are likely to be managed by neurodevelopmental and neurodisability paediatricians. Helpful resources are available for teachers as well as for individual patients and parents; Tourettes Action[41]
in the UK and the Tourette Association of America[42]
offer information and support. Patients with uncertain diagnosis, severe symptoms or complex presentations should be referred to a specialist clinic, where an in-depth assessment from a behavioural neurology point of view can be conducted in order to confirm the diagnosis (including comorbidities) and a targeted treatment plan can be formulated. Given that several comorbidities of chronic tic disorders are psychiatric, it is important to highlight the multidisciplinary nature of these assessments, which should ideally integrate neuropsychiatric and psychological input. The first step in the management process is psychoeducation, to be provided by a clinician who is knowledgeable about the complexities of the disorder and its evolving treatment. This should be extended to include the patient’s relatives, teachers, employers and the medical professionals involved, depending on the individual case[4]
.
The European Society for the Study of Tourette Syndrome (ESSTS) recently published the first European set of guidelines for the assessment and management of Tourette syndrome, which covers behavioural interventions, pharmacotherapy and surgical options for severe, treatment refractory cases[43]
,[44]
,[45]
,[46]
. These were followed by the Canadian guidelines for the evidence-based treatment of tic disorders[47]
,[48]
, which focus on the same treatment interventions.
Among the available behavioural strategies targeting tic symptoms, habit reversal training and exposure and response prevention appear to be the most promising approaches[49]
. Both methods are based on patients’ awareness of their premonitory urges in order to potentiate tic suppression. As part of habit reversal training, patients are encouraged to resist their urges for a specific tic by using a tailored competing response (a more comfortable/acceptable movement or sound), which replaces the actual tic[50]
. Over the past few years, an established North American group published the results of two large randomised controlled trials in children/adolescents[51]
and adults[52]
with Tourette syndrome or chronic tic disorders, showing that a comprehensive behavioural intervention for tics incorporating the habit reversal training component contributed to significant reduction in tic severity in around half of the patients. These promising results need to be replicated by trials conducted in other centres actively involved in clinical research on tic disorders. According to a recent national survey in the UK, behavioural treatments are preferred by patients but often not available (only accessed by 25% of patients, although desired by 75%)[53]
. Different cognitive-behavioural approaches can prove useful in the initial management of co-morbid obsessive compulsive symptoms and attention deficit and hyperactivity symptoms[54]
.
A comprehensive assessment of the nature and severity of individual tics, combined with evaluation of tic-related behavioural problems and overall impact on health-related quality of life, could prompt targeted pharmacotherapeutic intervention in specialist settings. Pharmacotherapy should be implemented in addition to psychoeducation and as an alternative/add-on to behavioural therapy for patients with clear impairment associated with their tics. The ESSTS recently published recommendations based on expert consensus on the indications to pharmacotherapy in patients with Tourette syndrome[44]
. These indications include presence of tics causing subjective discomfort (e.g. pain or injury), sustained problems in social life (e.g. isolation or bullying), emotional problems (e.g. reactive depression) or functional interference (e.g. in school or at work)[44]
.
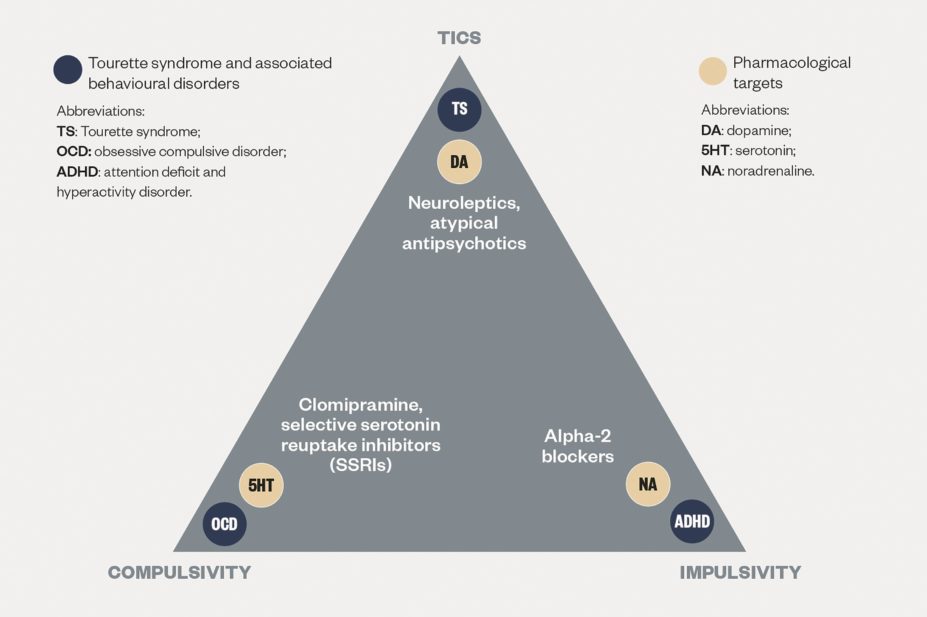
Agents belonging to a range of pharmacological classes have proven useful in different patients, depending on their clinical presentation and most prominent symptoms (see ‘Targeted treatments for symptoms of selected behavioural disorders’).
Unfortunately, evidence-based conclusions can only be drawn for older agents, which were tested in a sufficient number of double-blind randomised controlled trials. Therefore, practical recommendations often have to take into account the results of open-label studies and large case series[54]
. The clinical reality is that the European guidelines[44]
, based on expert consensus, place the atypical antipsychotics risperidone and aripiprazole as highest in the first-line treatments for tics, together with the alpha-2 agonist agent clonidine, which is prioritised by the Canadian guidelines [47]
, alongside guanfacine. With guanfacine extended-release recently receiving a European marketing licence, the latter becomes more relevant for the UK.
Over the past couple of decades, newer antidopaminergic agents (atypical antipsychotics) have increasingly been used as first-line options in place of older neuroleptics, based on their better tolerability profile (especially in terms of extrapyramidal adverse effects) and similar level of efficacy against tics. Among the atypical antipsychotics, risperidone (1–4mg daily) is the individual agent with the strongest level of evidence and features at the top of the list of European experts’ recommendations for the treatment of tics in children and adolescents[44]
. More recently, treating clinicians have also developed confidence about the first-line use of a different atypical antipsychotic, aripiprazole (5–10mg daily), which is characterised by a better tolerability profile thanks to its partial dopamine-agonist action. Evidence from open-label studies conducted over the past decade have shown that aripiprazole can lead to tic reduction in up to 80% of patients, while rates of discontinuation caused by adverse effects tend to be lower than 20%[44]
. The better tolerability profile of aripiprazole is particularly significant in terms of cardiovascular contraindications, which are prominent in neuroleptics (especially pimozide) and, to a lesser extent, other atypical antipsychotics[55]
. Qualitative studies have recently shown that aripiprazole is also preferred by patients[53]
. In terms of dose adjustments in different patient populations, the use of more than risperidone 2mg once daily or aripiprazole 5mg once daily would not be routinely endorsed by clinicians in the UK for use in children with Tourette syndrome, whereas adult patients often require higher doses.
Alpha-2 agonists such as clonidine (0.1–0.3mg daily) and guanfacine (1–4mg daily) are often used as first-line pharmacological options for young patients with tic disorders, based on a good level of evidence for efficacy and, importantly, tolerability (apart from dose-dependent hypotensive effects and mild sedation). The dose of clonidine is usually calculated depending on the child’s weight (3–5mcg/kg)[56]
. In consideration of the high rate of co-morbid ADHD in young patients with Tourette syndrome, the use of alpha-2 agonists can be particularly useful since their anti-noradrenergic action has been shown to be potentially effective also for attentional problems and hyperactivity[57]
. Likewise, antidopaminergic agents can be a useful addition in patients with tic disorders and co-morbid OCD treated with serotonergic agents as augmentation therapy (selective serotonin reuptake inhibitors or the tricyclic clomipramine). Finally, it should be noted that there is no evidence that stimulants necessarily exacerbate tics when used to treat co-morbid attention deficit and hyperactivity symptoms in patients with Tourette syndrome, especially if administered in lower doses and with gradual titration[58]
,[59]
.
Specialist interventions
It is recommended that patients with tic disorders are reviewed, at least on a yearly basis in specialist settings, where further management plans can be explored in case of a lack of response to first-line treatment options[60]
. Older generation antidopaminergic drugs such as the neuroleptic agents haloperidol (therapeutic range 0.25–15mg daily) and pimozide (1–6mg daily) are still considered among the most effective tic suppressants and have licensed indications for tic disorders. However, the benefits provided by these medications are often associated with significant adverse effects, including extrapyramidal and metabolic problems, as well as sedation and depression, which restrict their use to selected patients as second-line or third-line pharmacological options. In terms of alternative antidopaminergic agents, both monoamine presynaptic depletor tetrabenazine (25–200mg daily) and benzamides sulpiride and tiapride (2–10mg/kg daily) can be considered viable options, despite significant metabolic adverse effects, especially hyperprolactinaemia and weight gain. With regard to different pharmacological classes, antiepileptic drugs with GABA-ergic effects such as topiramate (50–200mg daily) have recently proven beneficial in small open-label and double-blind controlled trials, although further studies are necessary to confirm these initial findings.
Botulinum toxin injections have sometimes been used for the symptomatic treatment of isolated tics, with positive (but not permanent) results, especially in patients with dystonic tics. Finally, a few selected patients with severe and treatment-refractory Tourette syndrome have undergone the neurosurgical procedure of deep brain stimulation[61]
. Albeit far from being risk-free, this functional procedure is less invasive than ablative neurosurgery and implements a stereotactic approach to target subcortical structures thought to be involved in the pathophysiology of tic disorders (mainly globus pallidus-pars interna and thalamic ventromedian-parafascicular nucleus). In consideration of the heterogeneous nature of preliminary data on the use of deep brain stimulation for patients with Tourette syndrome, it is not surprising that existing national guidelines show a degree of variability in terms of both optimal targets and suitable candidates[62]
. At present, this procedure is still considered experimental and can only be recommended as a last resort in patients aged over 18 years. By shedding more light on the pathophysiology of tic disorders, it is likely that future research will open up new treatment avenues both in terms of pharmacological and non-pharmacological therapy.
Andrea E Cavanna is professor of neuropsychiatry at the school of life and health sciences, Aston Brain Centre, Aston University, and consultant in behavioural neurology at the department of neuropsychiatry, Birmingham and Solihull Mental Health Foundation NHS Trust, Birmingham, UK. Stefano Seri is professor of clinical neurophysiology and developmental neuropsychiatry at the school of life and health sciences, Aston Brain Centre, Aston University, Birmingham, UK.
Financial and conflicts of interest disclosure: Andrea Cavanna is a member of the Medical Advisory Board of the USA Tourette Syndrome Association and has received board membership fees and research grants from Eisai Pharmaceuticals and lectureship grants from Eisai Pharmaceuticals, UCB Pharma and Janssen-Cilag. Stefano Seri has received unrestricted educational grants from Eisai Pharmaceuticals, UCB Pharma and Beacon Pharmaceuticals Limited. No writing assistance was utilised in the production of this manuscript.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] Ganos C & Martino D. Tics and Tourette syndrome. Neurologic Clinics 2015;33:115–136. doi: 10.1016/j.ncl.2014.90.008
[2] de la Tourette G. Etude sur une affection nerveuse caracterisée par de l’incoordination motrice accompagnée d’écholalie et de coprolalie. Archives de Neurologie (Paris) 1885;9:19–42,158–200.
[3] Rickards H, Woolf I & Cavanna AE. Trousseau’s disease: A description of Gilles de la Tourette syndrome 12 years before 1885. Movement Disorders 2010;25:2285–2289. doi: 10.1002/mds.23202
[4] Cavanna AE & Seri S. Tourette’s syndrome. The BMJ 2013;347:f4964. doi: 10.1136/bmj.f4964
[5] Hallett M. Tourette syndrome: update. Brain and Development 2015;37:651–655. doi: 10.1016/j.braindev.2014.11.005
[6] Scharf JM, Miller LL, Gauvin CA et al. Population prevalence of Tourette syndrome: a systematic review and meta-analysis. Movement Disorders 2015;30:221–228. doi: 10.1002/mds.26089
[7] Stern JS, Burza S & Robertson MM. Gilles de la Tourette’s syndrome and its impact in the UK. Postgraduate Medical Journal 2005;81:12–19. doi: 10.1136/pgmj.2004.023614
[8] Ong MT, Mordekar SR & Seal A. 15 minute consultation: Tics and Tourette syndrome. Archives of Disease in Childhood: Education and Practice Edition 2015;(In press). doi: 10.1136/archdischild-2015-309138
[9] Robertson MM. The prevalence and epidemiology of Gilles de la Tourette syndrome. Part 1: The epidemiological and prevalence studies. Journal of Psychosomatic Research 2008;65:461–472. doi: 10.1016/j.psychores.2008.03.006
[10] Knight T, Steeves T, Day L et al. Prevalence of tic disorders: A systematic review and meta-analysis. Pediatric Neurology 2012;47:77–90. doi: 10.1016/j.pediatrneurol.2012.05.002
[11] Felling RJ & Singer HS. Neurobiology of Tourette syndrome: Current status and need for further investigation. Journal of Neuroscience 2011;31:12387–12395. doi: 10.1523/JNEUROSCI.0150-11.2011
[12] Cavanna AE, Stecco A, Rickards H et al. Corpus callosum abnormalities in Tourette syndrome: An MRI-DTI study of monozygotic twins. Journal of Neurology Neurosurgery and Psychiatry 2010;81:533–535. doi: 10.1136/jnnp.2009.173666
[13] Nagai Y, Cavanna AE & Critchley HD. Influence of sympathetic arousal on tics: Implications for a therapeutic behavioral intervention for Tourette syndrome. Journal of Psychosomatic Research 2009;67:599–605. doi: 10.1016/j.jpsychores.2009.06.004
[14] Ali F, Morrison KE & Cavanna AE. The complex genetics of Gilles de la Tourette syndrome: Implications for clinical practice. Neuropsychiatry 2013;3:321–330. doi: 10.2217/npy.13.25
[15] Madhusudan N & Cavanna AE. The role of immune dysfunction in the development of tics and susceptibility to infections in Tourette syndrome: a systematic review. Basal Ganglia 2013;3:77–84. doi: 10.1016/B978-0-12-411546-0.00001-9
[16] Cavanna AE & Rickards H. The psychopathological spectrum of Gilles de la Tourette syndrome. Neuroscience and Biobehavioral Reviews 2013;37:1008–1015. doi: 10.1016/j.neubiorev.2012.10.011
[17] Martino D, Cavanna AE, Robertson MM et al. Prevalence and phenomenology of eye tics in Gilles de la Tourette syndrome. Journal of Neurology 2012;259:2137–2140. doi: 10.1007/s00415-012-6470-1
[18] Eddy CM & Cavanna AE. ‘It’s a curse!’: Coprolalia in Tourette syndrome. European Journal of Neurology 2013;20:1467–1470. doi: 10.1111/ene.12207
[19] Crossley E & Cavanna AE. Sensory phenomena: Clinical correlates and impact on quality of life in adult patients with Tourette syndrome. Psychiatry Research 2013;209:705–710. doi: 10.1016/j.psychres.2013.04.019
[20] Crossley E, Seri S, Stern JS et al. Premonitory urges for tics in adult patients with Tourette syndrome. Brain and Development 2014;36:45–50. doi: 10.1016/j.braindev.2012.12.010
[21] Cavanna AE, Robertson MM & Critchley HD. Catatonic signs in Gilles de la Tourette syndrome. Cognitive and Behavioral Neurology 2008;21:34–37. doi: 10.1097/WNN.0b013e318165a9cf
[22] Hassan N & Cavanna AE. The prognosis of Tourette syndrome: Implications for clinical practice. Functional Neurology 2012;27:23–27. PMCID: PMC3812751
[23] Eddy CM & Cavanna AE. Altered social cognition in Tourette syndrome: Nature and implications. Behavioural Neurology 2013;27:15–22. doi: 10.3233/BEN-120298
[24] Eddy CM & Cavanna AE. On being your worst enemy: An investigation of socially inappropriate symptoms in Tourette syndrome.Journal of Psychiatric Research 2013;47:1259–1263. doi: 10.1016/j.jpsychires.2013.05.019
[25] Freeman RD, Fast DK, Burd L et al. An international perspective on Tourette syndrome: Selected findings from 3500 individuals in 22 countries. Developmental Medicine and Child Neurology 2000;42:436–447. doi: 10.1111/j.1469-8749.2000.tb00346.x
[26] Khalifa N & Von Knorring A-L. Tourette syndrome and other tic disorders in a total population of children: Clinical assessment and background. Acta Paediatrica 2005;94:1608–1614. doi: 10.1111/j.1651-2227.2005.tb01837.x
[27] Eddy CM, Cavanna AE, Gulisano M et al. The effects of comorbid obsessive-compulsive disorders and attention-deficit hyperactivity disorder on quality of life in Tourette syndrome. Journal of Neuropsychiatry and Clinical Neurosciences 2012;24:458–462. doi: 10.1176/appi.neuropsych.11080181
[28] Eddy CM, Rickards H, Critchley HDet al. A controlled study of personality and affect in Tourette syndrome. Comprehensive Psychiatry 2013;54:105–110. doi: 10.1016/j.comppsych.2012.07.004
[29] Frank MC, Piedad J, Rickards H et al. The role of impulse control disorders in Tourette syndrome: an exploratory study. Journal of the Neurological Sciences 2011;310:276–278. doi: 10.1016/j.jns.2011.06.032
[30] Cavanna AE, Schrag A, Morley D et al. The Gilles de la Tourette Syndrome-Quality of Life scale (GTS-QOL): Development and validation. Neurology 2008;71:1410–1416. doi: 10.1212/01.wnl.0000327890.02893.61
[31] Cavanna AE, Luoni C, Selvini C et al. The Gilles de la Tourette Syndrome-Quality of Life Scale for Children and Adolescents (GTS-QOL-C&A): Development and validation of the Italian version. Behavioural Neurology 2013;27:95–103. doi: 10.3233/BEN-120274
[32] Cavanna AE, Luoni C, Selvini C et al. Disease-specific quality of life in young patients with Tourette syndrome. Pediatric Neurology 2013;48:111–114. doi: 10.1016/j.pediatrneurol.2012.10.006
[33] Cavanna AE, David K, Bandera V et al. Health-related quality of life in Gilles de la Tourette syndrome: A decade of research. Behavioural Neurology 2013;27:83–93. doi: 10.3233/BEN-120296
[34] American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Washington, DC: American Psychiatric Association 2013.
[35] Woods DW & Thomsen PH. Tourette and tic disorders in ICD-11: standing at the diagnostic crossroads. Revista Brasileira de Psiquiatria 2014;36(1):51–58. doi: 10.1590/1516-4446-2013-1274
[36] Tourette Syndrome Classification Study Group. Definitions and classification of tic disorders. Archives of Neurology 1993;50:1013–1016. doi: 10.1001/archneur.1993.00540100012008
[37] Worbe Y, Mallet L, Golmard JL et al. Repetitive behaviours in patients with Gilles de la Tourette syndrome: Tics, compulsions, or both? PLoS One 2010;5:e12959. doi: 10.1371/journal.pone.0012959
[38] Robertson MM. A personal 35 year perspective on Gilles de la Tourette syndrome: prevalence, phenomenology, comorbidities, and coexistent psychopathologies. Lancet Psychiatry 2015;2:68–87. doi: 10.1016/S2215-0366(14)00132-1
[39] Termine C, Selvini C, Balottin U et al. Self-, parent-, and teacher-reported behavioural symptoms in young people with Tourette syndrome: A case-control study. European Journal of Paediatric Neurology 2011;15:95–100. doi: 10.1016/j.ejpn.2011.01.002
[40] Cavanna AE, David K, Robertson MM et al. Predictors during childhood of future health-related quality of life in adults with Gilles de la Tourette syndrome. European Journal of Paediatric Neurology 2012;16:605–612. doi: 10.1016/j.ejpn.2012.02.004
[41] Tourettes Action. www.tourettes-action.org.uk (accessed January 2016).
[42] Tourette Association of America. www.tourette.org (accessed January 2016).
[43] Cath DC, Hedderly T, Ludolph AG et al. ESSTS Guidelines Group. European clinical guidelines for Tourette syndrome and other tic disorders. Part I: Assessment. European Child and Adolescent Psychiatry 2011;20:155–171. doi: 10.1007/s00787-011-0164-6
[44] Roessner V, Plessen KJ, Rothenberger A et al. ESSTS Guidelines Group. European clinical guidelines for Tourette syndrome and other tic disorders. Part II: Pharmacological treatment. European Child and Adolescent Psychiatry 2011;20:173–196. doi: 10.1007/s00787-011-0163-7
[45] Verdellen C, van de Griendt J, Hartmann A et al; ESSTS Guidelines Group. European clinical guidelines for Tourette syndrome and other tic disorders. Part III: Behavioural and psychosocial interventions. European Child and Adolescent Psychiatry 2011;20:197–207. doi: 10.1007/s00787-011-0167-3
[46] Muller-Vahl KR, Cath DC, Cavanna AE et al. The ESSTS Guidelines Group. European clinical guidelines for Tourette syndrome and other tic disorders. Part IV: Deep brain stimulation. European Child and Adolescent Psychiatry 2011;20:209–217. doi: 10.1007/s00787-011-0166-4
[47] Pringsheim T, Doja A, Gorman D et al. Canadian guidelines for the evidence-based treatment of tic disorders: Pharmacotherapy. Canadian Journal of Psychiatry 2012;57:133–143. PMID: 22397999
[48] Steeves T, McKinlay D, Gorman D et al. Canadian guidelines for the evidence-based treatment of tic disorders: Behavioural therapy, deep brain stimulation and transcranial magnetic stimulation. Canadian Journal of Psychiatry 2012;57:144–151. PMID: 22398000
[49] Frank M & Cavanna AE. Behavioural treatments for Tourette syndrome: An evidence-based review. Behavioural Neurology 2013;27:105–117. doi: 10.3233/BEN-120309
[50] Dutta N & Cavanna AE. The effectiveness of habit reversal therapy in the treatment of Tourette syndrome and other chronic tic disorders: a systematic review. Functional Neurology 2013;28:7–12. doi: 10.11138/FNeur/2013.28.1.007
[51] Piacentini J, Woods DW, Scahill Let al. Behavior therapy for children with Tourette disorder: a randomized controlled trial. Journal of the American Medical Association 2010;303:1929–1937. doi: 10.1001/jama.2010.607
[52] Wilhelm S, Peterson AL, Piacentini J et al. Randomized trial of behavior therapy for adults with Tourette syndrome. Archives of General Psychiatry 2012;69:795–803. doi: 10.1001/archgenpsychiatry.2011.1528
[53] Cuenca J, Glazebrook C, Kendall T et al. Perceptions of treatment for tics among young people with Tourette syndrome and their parents: A mixed methods study. BMC Psychiatry 2015;15:46. doi: 10.1186/s12888-015-0430-0
[54] Waldon K, Hill S, Termine C et al. Trials of pharmacological interventions for Tourette syndrome: a systematic review. Behavioural Neurology 2013;26:265–273. doi: 10.3233/BEN-2012-120269
[55] Gulisano M, Calì PV, Cavanna AE et al. Cardiovascular safety of aripiprazole and pimozide in young patients with Tourette syndrome. Neurological Sciences 2011;32:1213–1217. doi: 10.1007/s10072-011-0678-1
[56] Taylor D, Paton C & Kapur S. The Maudsley Prescribing Guidelines in Psychiatry; 12th Edition. Wiley-Blackwell: 2015.
[57] Bloch MH, Panza KE, Landeros-Weisenberger A et al. Meta-analysis: Treatment of attention-deficit hyperactivity disorder in children with comorbid tic disorders. Journal of the American Academy of Child and Adolescent Psychiatry 2009;48:884–893. doi: 10.1097/CHI.0b013e3181b26e9f
[58] Erenberg G. The relationship between Tourette syndrome, attention deficit hyperactivity disorder, and stimulant medication: a critical review. Semin Pediatr Neurol 2005;12:217–221. doi: 10.1016/j.spen.2005.12.003
[59] Robertson MM. Attention deficit hyperactivity disorder, tics and Tourette’s syndrome: the relationship and treatment implications. Eur Child Adolesc Psychiatry 2006;15:1–11. PMID: 16514504
[60] Rickards H, Wood C & Cavanna AE. Hassler and Dieckmann’s seminal paper on stereotaxic thalamotomy for Gilles de la Tourette syndrome: Translation and critical reappraisal. Movement Disorders 2008;23:1966–1972. doi: 10.1002/mds.22238
[61] Vandewalle V, van der Linden C, Groenewegen HJ et al. Stereotactic treatment of Gilles de la Tourette syndrome by high frequency stimulation of thalamus. The Lancet 1999;353:724. doi: 10.1016/S0140-6736(98)05964-9
[62] Cavanna AE, Eddy CM, Mitchell R et al. An approach to deep brain stimulation for severe treatment-refractory Tourette syndrome: The UK perspective. British Journal of Neurosurgery 2011;25:38–44. doi: 10.3109/02688697.2010.534200
You might also be interested in…

Medicines shortages and slow approvals put ‘significant burden’ on pharmacists, says report
Diltiazem use with anticoagulants associated with higher risk of bleeding compared with metoprolol in older people, study finds