Prof P. Motta / Dept of Anatomy / University. Science Photo Library
Osteoporosis is a long-term condition, characterised by low bone mass and micro-architectural bone deterioration, leading to an increased risk of fracture[1]
. The three most common fracture sites are the wrist, spine (vertebrae) the hip[2]
.
Around one in two women and one in five men aged over 50 years will break a bone; the vast majority of which will be the result of a fall[3],[4],[5]
. A third of people aged over 65 years fall at least once per year, and this increases to half of those aged 80 years and over[3],[6],[7]
. Falls can have a significant impact on an older person — they are a cause of pain, loss of confidence and loss of independence[3],[4],[6]
. Around 53% of patients with a hip fracture are unable to live independently and 28% will die within a year of fracture[4],[8]
. Furthermore, fragility fractures (fractures caused by falls or trauma from standing height or lower) cost the NHS an estimated £4.4bn per year — around half of which are owing to hip fractures[4],[9]
.
Although common in older people, osteoporosis often remains undiagnosed until after a fall or fracture. With an ageing population in the UK, it is estimated that the number of osteoporotic fractures will double over the next 50 years if changes are not made to current practice[4],[9]
. As such, older people presenting with a fragility fracture should have their bone health assessed.
Pharmacists and other healthcare professionals should understand bone health in the context of the older person in order to identify those who are at risk and to promote preventative measures (including for secondary fractures). This article describes the diagnosis and management of osteoporosis, including considerations for patients with comorbidities.
Pathophysiology
Bone is an active tissue that constantly remodels in response to microfractures that occur from everyday wear and tear. Bone remodelling, where old bone is removed and replaced by new bone, is controlled by three major cell types: osteoblasts, osteoclasts and osteocytes. In the normal adult skeleton there is a balance between new bone being made by osteoblasts and old bone being resorbed by osteoclasts[10]
. However, in osteoporosis, bone loss occurs because bone resorption is greater than bone formation.
Peak bone mass typically occurs between the ages of 25 years and 35 years, and normal bone density loss is 1% per year after the age of 40 years in both men and women[10],[11]
. Bone mineral density (BMD) is a measure of the amount of minerals (mainly calcium and phosphorous) contained in a certain volume of bone[12]
. In women, menopause accelerates the decline in BMD to 3–4% per year[13]
. Some comorbidities, as well as medicines, can also influence BMD over time[14]
(see Table 1).
Diagnosis
Risk factors
Osteoporosis is defined by the World Health Organization (WHO) as having BMD of ≥2.5 standard deviations below the average value for a young adult (i.e. T-score ≤–2.5)[1],[15]
. There are several risk factors that affect BMD (see Table 1).
| Risk factors | Description |
|---|---|
| Source: Postgrad Med J [16] | |
| Gender and age | Increased risk of osteoporotic fractures in postmenopausal women. |
| Body weight | Low body mass index is associated with increased fracture risk. |
| Physical inactivity/sedentary lifestyle | Physical loading on to skeletal bones and physical activity helps to increase bone mineral density (BMD) and decrease fracture risk. |
| Extraskeletal risk factors | Increasing age, postural instability, orthostatic hypotension, sarcopenia, recurrent falls, poor coordination and unsteady gait have been associated with increased fracture risk in people with osteoporosis. |
| Alcohol consumption and cigarette smoking | Alcohol affects calcium metabolism and is considered significant if alcohol intake is >3 units of alcohol per day. Smoking is associated with reduced BMD. |
| Medicines | Long-term use of steroids has been shown to decrease BMD, predisposing individuals to osteoporosis. Other medicines that contribute to osteoporosis include, but are not limited to:
Medicines that cause drowsiness or affect balance further increase extraskeletal risk factors (e.g. sedatives, benzodiazepines, anticholinergics, antihypertensives, opioids and antidepressants). |
| Secondary causes of osteoporosis | Inflammatory bowel disease (malabsorption), rheumatoid arthritis, malignancy, endocrine disorders (type 1 diabetes mellitus, Cushing’s syndrome, hyperthyroidism, hypogonadism, hyperparathyroidism) and premature menopause (<45 years old) are conditions associated with an increased risk of osteoporosis. |
| Previous fracture or history of parent hip fracture | Any previous vertebral fracture (even those detected via radiographic imaging alone) and hip fractures, including the history of a parent with a hip fracture, are considered strong risk factors. |
The important risk factors mentioned in Table 1 are incorporated into online tools that calculate a personalised ten-year probability of major osteoporotic or hip fracture for each individual. The most commonly used assessment tool in practice is FRAX® (which was developed by the WHO Collaborating Centre for Metabolic Bone Disease at the University of Sheffield), although QFracture® is another alternative. Both are freely available online.
FRAX allows further triaging of patients to either:
- Low risk of falls and fractures — requiring lifestyle modification and adequate nutritional intake of calcium and vitamin D;
- Intermediate risk of falls and fractures — suggesting a dual-energy X-ray absorptiometry (DEXA) scan to assess their BMD. If the BMD T-score diagnosis is osteoporosis then treatment should be started. DEXA scans are not routinely used above the age of 75 years as it is more cost effective to treat this group of patients with a high inherent risk of fracture;
- High risk of falls and fractures — can be considered for treatment without the need for BMD assessment.
An example of how FRAX can be used to calculate fracture risk and assessment threshold is shown in Figure 1 for a hypothetical 86-year-old female (weight 56kg and height 152cm) with no other risk factors and no previous calculation of BMD. However, it is important to note that if major osteoporotic fracture and hip fracture risk is recalculated using femoral neck BMD, FRAX may underestimate the vertebral fracture risk probability.
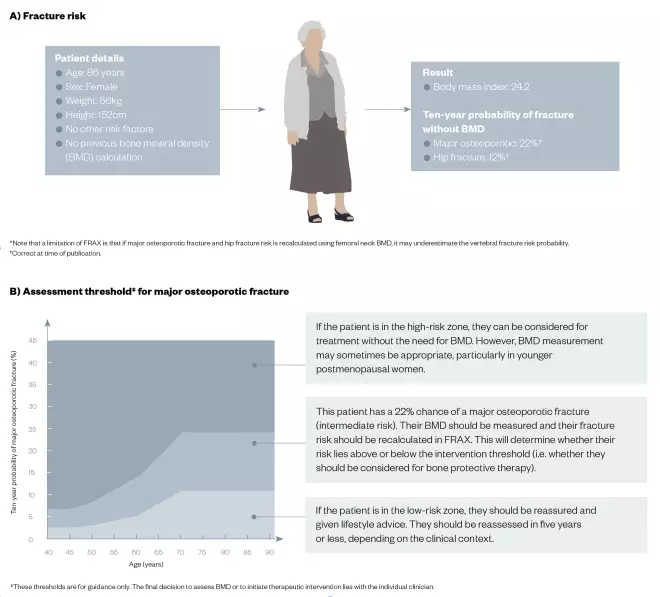
Figure 1: Using the FRAX® calculation tool for fracture risk and the assessment threshold for major osteoporotic fracture in a hypothetical patient
Source: JL / The Pharmaceutical Journal
By inputting the following patient details into the online FRAX* calculation tool, healthcare professionals can calculate (A) risk of fracture because of weak bones, and (B) assessment threshold for major osteoporotic fracture.
Imaging
A DEXA scan is the most commonly used imaging modality in diagnosing osteoporosis; it measures an individual’s BMD by estimating the amount of bone at certain sites (most commonly the thoracolumbar spine [usually T4 to L4], forearm and hip).
Other uses of a DEXA scan include[4]
:
- Monitoring BMD following treatment as per recommendations;
- Differentiating osteoporosis from osteopenia (where BMD is lower than average, but not enough to be diagnosed as osteoporosis).
Using the T-scores from a DEXA scan, patients can be classified based on the WHO diagnosis of osteoporosis[15],[17]
(see Table 2).
| T-score (standard deviations) | Diagnosis |
|---|---|
| ≥–1.0 | Normal bone density |
| Between –1.0 and –2.5 | Osteopenia |
| –2.5 | Osteoporosis |
Management
Once osteoporosis has been diagnosed, or the patient has been assessed as requiring treatment for bone protection, there are important questions to consider before treatment is started:
- Is the patient aware of the goals of treatment?
- Does the patient want treatment?
- Are there any comorbidities or cultural aspects that may affect choice of treatment?
- Can the patient take oral medicine and can they swallow safely?
- Can the patient remain upright for 30–60 minutes?
- Does the patient have cognitive impairment?
- Does the patient manage their own medicines and can they remember how to take them?
- Does the patient have renal impairment?
- Where does the patient reside?
- Is the patient bedbound?
Obtaining a thorough and detailed patient history will help guide choice of treatment. The decision to treat should be made on an individual basis and only once there has been open dialogue between the healthcare professional and patient about the benefits and risks of the treatments available. Online decision support tools are available, with the commonly used ones supplied by the National Institute for Health and Care Excellence (NICE) and the Mayo Clinic.
Diet and lifestyle
Bone health can be improved by having a healthy diet (including adequate calcium and vitamin D), exercising regularly, stopping smoking and reducing alcohol intake. In the case of older people, assessing and mitigating risk of falls is also essential. Most osteoporosis services have close links with falls prevention services where further assessment can be undertaken.
For patients at low risk of fracture, lifestyle advice should be offered with a follow-up within five years[6],[18]
. For those at high risk of fracture, lifestyle advice is still relevant but the type of exercise recommended must be tailored to the individual to ensure they do not increase the risk of falls or fractures (see Box 1).
Box 1: The evidence base for lifestyle advice in patients with osteoporosis
Balanced diet
There is no evidence that specific diets reduce fracture risk, but the National Institute for Health and Care Excellence recommends having a balanced diet[18]
.
Smoking
Smoking is a risk factor for fragility fracture. There is indirect evidence that suggests stopping smoking may reduce the risk of osteoporotic fragility fracture in women[19]
.
Alcohol
Advice is to drink within recommended limits, because alcohol is a dose-dependent risk factor for fragility fractures. It also appears to have some effect on osteoblasts and slowing bone turnover, although the exact mechanisms behind this are unclear[6]
.
Regular exercise
The aim is to improve muscle strength and evidence shows that some tailored exercise, such as single-leg standing or weight training, can slow the decline of bone mineral density[6]
. Weight-bearing exercise, where you work against gravity, has been advocated as the best exercise for bone health[20]
. Physical inactivity is often seen in older people and, where possible, mobility should be maintained with the help of physiotherapists. Balance training, stretching, endurance exercise and progressive strengthening exercises should all be considered to reduce risk of fractures caused by falls[6]
. The Royal Osteoporosis Society has published a reference for healthcare professionals to help advise on correct movements and exercises to promote bone strength and reduce fracture and falls risk[21]
.
Maintain a healthy weight
Adults with a low body mass index (BMI <20kg/m2) are at increased risk of fracture[6]
and should be encouraged to maintain a BMI of 20–25kg/m2. Low body weight is often seen in older, more frail patients and so the role of the dietitian is crucial.
Calcium is the major component in bone and is required for bone mineralisation[10]
. Vitamin D increases the intestinal absorption of calcium and also has a role in bone mineralisation[10]
. Maintaining adequate calcium intake (700mg/day) and vitamin D status (>50 nanomol/L), either by diet or supplements, is an essential part of bone health[4]
. A simple dietary calculator
could help in this area.
Increasing calcium intake has been shown to have some small effects in increasing BMD[18],[22]
, although evidence of its use alone in reducing fracture risk has not been demonstrated[4]
. If calcium intake is inadequate, patients should be advised to take supplementation of at least 1000mg calcium daily[4],[6],[18]
. There have been some concerns associated with the use of calcium relating to increased cardiovascular risk but there is little evidence for a significant association[4],[23]
. However, calcium can lead to increased risk of renal calculi and patients who take calcium supplements often complain of gastrointestinal symptoms, particularly constipation, which can reduce concordance[24]
.
In 2016, the Scientific Advisory Committee on Nutrition published recommendations for vitamin D, which included 400 units of vitamin D per day for adults of all ages[25]
. However, in postmenopausal women and men aged over 50 years, a daily dose of 800 units of vitamin D is recommended[4]
. People at high risk of vitamin D deficiency include older people, people on restricted diets (e.g. vegan/vegetarian) and people with dark pigmentation skin.
It is also important to note that all the clinical trials involving treatments for osteoporosis have included supplementation with calcium and vitamin D.
Pharmacological
There is no distinction in treatment and preventative strategies in managing patients at high risk of fracture. Bisphosphonates are the mainstay of osteoporosis management, but other options include denosumab, raloxifene and teriparatide (see Table 3). Calcitriol is used in patients with chronic kidney disease (CKD).
| Bone-sparing agent | Anti-fracture efficacy | Licensed uses | Dose |
|---|---|---|---|
| Alendronate | VF, NVF, HF Grade A evidence
|
|
|
|
| ||
|
| ||
| Denosumab | VF, NVF, HF Grade A evidence |
|
|
| Ibandronate | VF, NVF* Grade A evidence |
|
|
| Raloxifene | VF Grade A evidence |
|
|
| Risedronate | VF, NVF, HF Grade A evidence |
|
|
| Teriparatide | VF, NVF Grade A evidence |
|
|
| Zoledronate | VF, NVF, HF Grade A evidence |
|
|
HF: hip fracture; IV: intravenous; NVF: non-vertebral fracture; VF: vertebral fracture. *Grades of evidence are taken from the National Osteoporosis Guideline Group’s clinical guideline for the treatment and prevention of osteoporosis †In a subset of patients only (post-hoc analysis) ‡ Licensed maximum duration of treatment was increased from 18 to 24 months through safety profile rather than any additional clinical benefit. Therefore locally, duration of treatment may remain at 18 months Sources: National Osteoporosis Guideline Group[4] | |||
Bisphosphonates
These drugs are potent inhibitors of osteoclast-mediated bone resorption. They are synthetic analogues of pyrophosphate, which is an endogenous regulator of bone mineralisation, and thus have a high affinity for the bone matrix[26]
. There are two types of bisphosphonate — nitrogen-containing and non-nitrogen-containing — and each type has a distinct mechanism of action. The non-nitrogen-containing bisphosphonates (e.g. clodronate and etidronate) inhibit bone resorption by generating cytotoxic analogues of adenosine triphosphate that interfere with mitochondrial function and induce osteoclast apoptosis[27]
. The more potent nitrogen-containing bisphosphonates (e.g. alendronate, ibandronate, risedronate, pamidronate and zoledronate) act by inhibiting farnesyl pyrophosphate synthase in the mevalonate pathway (a biosynthetic pathway responsible for the production of cholesterol), thus preventing the prenylation and activation of small guanosine triphosphatases that are essential for the activity and survival of osteoclasts[27]
. This inhibition promotes the detachment of the osteoclast from the bone and leads to a reduction in bone resorption[27]
.
Owing to poor oral absorption (<1%), counselling is imperative for effective oral bisphosphonate use[28]
(see Box 2). Absorption is further reduced when the drugs are taken with food or with calcium- or iron-containing drugs, antacids, tea, coffee or fruit juice. Therefore, the recommendation is to take oral bisphosphonates with a full glass of water (but not mineral water) at least 30 minutes (when taking alendronate and risedronate) to an hour (when taking ibandronate) before breakfast, or before taking any other medication[29],[30],[31]
. Calcium-containing products or antacids should be taken at least two hours after the bisphosphonate. Taking the medicine before breakfast is necessary to ensure maximum absorption after the overnight fasting state. It is also advised that patients stand or sit upright for at least 30–60 minutes after administration, depending on the bisphosphonate taken[29],[30],[31]
. This advice helps to minimise the risk of oesophageal adverse effects associated with bisphosphonates (e.g. difficult and painful swallowing, chest pain or heartburn). In older patients who present with swallowing difficulties, poor oral intake or dehydration, it is important to consider if they will be able to comply with the administration instructions.
Box 2: Counselling points for oral bisphosphonate use
- Oral bisphosphonates should be taken after an overnight fast, at least 30 minutes (or 60 minutes in the case of ibandronate) before first food or drink (other than water) and any other medicines (including calcium supplements);
- The patient should be sitting or standing, and swallow the tablet whole with a glass of plain water. The volume of water should be:
- The patient should not lie down for 30 minutes (or 60 minutes in the case of ibandronate) after taking the tablet.
Oral bisphosphonates are contraindicated in patients who cannot remain upright for at least 30–60 minutes. Alendronate and oral ibandronate are contraindicated in those who have abnormalities of the oesophagus or have delayed oesophageal emptying (e.g. strictures or severe dysmotility)[31],[32]
; risedronate should also given be with caution in such patients[30]
. In patients with known Barrett’s oesophagus, the benefits and potential risks of alendronate and risedronate should be considered on an individual basis[29],[30]
, while oral ibandronate should be used with caution in these patients[31]
. All bisphosphonates should be used with caution in patients with other upper gastrointestinal disorders[33]
.
The intravenous (IV) infusion option of zoledronate may be suitable for patients who are unable to tolerate oral regimens; cannot sit upright for 30–60 minutes; or have poor concordance, memory problems, or anatomical or motility disorders of the upper gastrointestinal tract. It is important to be well hydrated before IV administration of bisphosphonates and, as such, patients are advised to drink plenty of water before treatment. Zoledronate infusion should be given over at least 15 minutes because rapid infusion can lead to acute kidney failure[34]
.
Bisphosphonates undergo renal clearance by glomerular filtration; the fraction that is not absorbed into bone is excreted, unchanged, in urine[27]
. Bisphosphonates can be used in patients with mild-to-moderate renal impairment; however, they are contraindicated in patients with a creatinine clearance (measured using the Cockroft–Gault formula) of <30–35mL/min, depending on the bisphosphonate, owing to an increased risk of kidney failure[29],[30],[31],[34]
. Therefore, patients who are prescribed bisphosphonates but have impaired kidney function (e.g. older patients, or those on nephrotoxic or diuretic therapy) require careful monitoring.
The most common side effects associated with oral bisphosphonates include upper gastrointestinal symptoms, bowel disturbance, headaches and musculoskeletal pain[33]
. Risedronate may be associated with fewer upper gastrointestinal events than alendronate and may be an appropriate switch if the patient is intolerant to alendronate. This is defined as persistent upper gastrointestinal disturbance that is severe enough to discontinue treatment, despite following administration instructions correctly[32]
. IV bisphosphonates are associated with an acute phase reaction characterised by influenza-like illness that usually only occurs after the first injection and is short lived[34]
.
Rarer side effects that pharmacists should monitor include osteonecrosis of the jaw (ONJ) and atypical femoral fractures (AFF) (see Figure 2). ONJ incidence in patients with osteoporosis has been estimated to be <0.1% (0–10 cases per 10,000 patients), with a UK study estimating alendronate-associated ONJ incidence to be 4.3 cases per 10,000 drug–patient years[35]
. AFF incidence has been reported to be up to 5 cases per 10,000 patients treated with osteoporosis medicine[4]
. Osteonecrosis of the external auditory canal is very rare, with incidence of fewer than 1 case per 10,000 patients treated with bisphosphonates for two or more years[36]
. Oral bisphosphonates have been associated with causing oesophageal cancer; in 2014, the Medicines and Healthcare products Regulatory Agency advised that an association cannot be excluded[37]
. Uveitis has also been reported in retrospective studies, particularly with zoledronate[6],[38]
. Some clinical trial data have suggested an increased risk of atrial fibrillation for zoledronate and possibly for alendronate[39]
. Pharmacists and healthcare professionals should perform certain checks before initiating treatment with bisphosphonates (see Box 3).

Figure 2: Medicines and Healthcare products Regulatory Agency alerts/drug safety updates associated with bisphosphonate and denosumab use
Sources: Medicines and Healthcare Products Regulatory Agency[36],[40],[41],[42],[43],[44],[45]
Box 3: Checks to perform before initiating treatment with bisphosphonates
- Is a bisphosphonate indicated?
- Does the patient have abnormalities of the oesophagus/Barrett’s oesophagus?
- Does the patient have difficulty swallowing?
- Does the patient have stomach problems?
- Patients with mild indigestion may be able to tolerate oral bisphosphonates, but should seek medical advice if it becomes problematic. If the patient has had a stomach ulcer in the past six months, oral treatment is not appropriate;
- Does the patient have cognitive impairment?
- If so, will they be able to follow instructions?
- Are there carers available who may be able to help?
- Is the patient able to remain upright for 30–60 minutes after taking oral bisphosphonates?
- What is the appropriate formulation for the patient?
- What is the patient’s renal function (measured by creatine clearance using the Cockroft–Gault formula)?
- What is the patient’s adjusted calcium and vitamin D serum level?
- Does the patient have good dental hygiene?
- Has the patient used a bone-sparing agent previously?
- If so, what were the reasons for cessation?
Denosumab
This is a fully human monoclonal antibody to the receptor activator of nuclear factor kappa-B ligand (RANKL), an osteoclast differentiating factor. It binds with high affinity to the cytokine RANKL, which inhibits its action; in turn, osteoclast recruitment, maturation and development are inhibited. This causes a reduction in bone resorption.
Denosumab is recommended as primary prevention in postmenopausal women at increased risk of fractures who are:
- Unable to comply with the administration instructions, or are intolerant of oral bisphosphonates, or in whom bisphosphonates are contraindicated;
- And who also have a set combination of a given T-score, age and number of independent risk factors[46]
.
NICE also recommends denosumab as a treatment option for secondary prevention in postmenopausal women who are unable to comply with the administration instructions, or have an intolerance or contraindication to oral bisphosphonates[46]
.
Denosumab use in men with osteoporosis has been extended based on a bridging study, but currently it is not endorsed by NICE[47]
. Updated guidance on non-bisphosphonates for treating osteoporosis is currently in development by NICE.
Side effects associated with denosumab use include skin infection (mainly cellulitis) and hypocalcaemia. Hypocalcaemia is a contraindication to treatment with denosumab and risk increases in those with renal impairment. Prior to treatment, adjusted serum calcium and vitamin D levels should be measured and adequate supplementation provided. The National Osteoporosis Guideline Group (NOGG) advises to check calcium levels within two weeks of the initial dose if a patient is predisposed to hypocalcaemia (i.e. patients with a creatinine clearance < 30mL/min) or if symptoms of hypocalcaemia are suspected (e.g. if there are muscle spasms, numbness, muscle cramps and confusion)[4]
.
As with the bisphosphonates, ONJ and AFF can rarely occur in patients treated with denosumab. The summary of product characteristics for densoumab (Prolia®; Amgen, UK) reports that 13 cases of ONJ were observed in 4,450 patients (0.3%) over seven years of an extended phase III clinical trial[35]
(see Figure 2).
Good concordance is important to ensure treatment efficacy of denosumab. Unlike the bisphosphonates, the effects of denosumab on bone resorption do not persist once treatment is stopped. It has been reported that soon after denosumab is stopped, rapid bone loss occurs and the bone-protective effects disappear[48]
. Having regular six-monthly treatment is critical — patients should be encouraged to attend appointments and have the necessary blood work completed. Treatment with denosumab is often initiated in secondary care but can often be transferred to the patient’s GP via a local shared care agreement. This allows greater convenience and flexibility for the patient, but it is important that pharmacists make all the appropriate clinical checks before prescribing or supplying.
Raloxifene
This drug is a selective oestrogen receptor modulator that inhibits bone resorption. Although it has been shown to reduce vertebral fracture risk, the same benefits have not been demonstrated in non-vertebral and hip fractures[4]
. NICE does not recommend raloxifene as a treatment option for the primary prevention of fragility fractures in postmenopausal women[49]
. However, it is approved for use as an alternative treatment for secondary prevention of fragility fractures in postmenopausal women who are unable to comply with the administration instructions, or have a contraindication/intolerance of oral bisphosphonates and have a combination of a given T-score, age and number of independent clinical risk factors for fracture, as set out in the NICE guideline[50]
.
Side effects include leg cramps, oedema and vasomotor symptoms (with reported incidence between 1 in 10 people to 1 in 100 people)[51]
. Raloxifene is contraindicated in women of childbearing age, who have a history of venous thromboembolism or unexplained uterine bleeding, hepatic impairment and severe renal impairment. It should be used with caution in women who have a history of stroke or who have risk factors of stroke[38]
. In the first few months of treatment, there is a small increase in the risk of venous thromboembolism (3.2 cases per 1,000 patient years) and fatal stroke (incidence reported as 2.2 per 1,000 women per year for raloxifene vs. 1.5 per 1,000 women per year for placebo)[51]
.
Teriparatide
This drug is recombinant human parathyroid hormone used in severe osteoporosis under specialist guidance. It works differently to the other treatment modalities owing to its anabolic effect on bone by stimulating bone formation by osteoblasts.
Teriparatide is contraindicated in patients with hypercalcaemia, metabolic bone diseases other than osteoporosis, severe renal impairment, prior radiation to the skeleton, or malignant disease effecting bone[52]
. Side effects include headache, nausea, dizziness and postural hypotension[38]
. As it is delivered by a daily subcutaneous injection, patients need to be appropriately counselled and consideration must be given to the older patient who may find it difficult to self-administer.
It has been available for use in women since it was licensed; in June 2018, NHS England also commissioned its use in men[53]
.
Monitoring and duration of treatment
During treatment, monitoring is essential to ensure efficacy and safety, but also to encourage concordance (see Table 4).
| Bone-sparing agent | Specific monitoring required | General monitoring |
|---|---|---|
| Oral bisphosphonate |
|
|
| Intravenous bisphosphonate |
| |
| Denosumab |
|
Most of the pivotal clinical trials involving bisphosphonates have been limited to three years and so extending treatment beyond this time is based on limited evidence from extension studies in postmenopausal women[4]
. The long-term side effects, particularly ONJ and atypical femoral fracture, have raised questions about optimal duration of therapy. As bisphosphonates are retained in bone for a relatively long time, there have been suggestions that patients could have a treatment break. Current advice from NOGG recommends reviewing treatment after five years of oral bisphosphonates and three years for IV zoledronate. Fracture risk should be reassessed during this treatment review, including a repeat DEXA scan after three years (for zoledronate) or five years (oral bisphosphonates). There is no evidence to guide treatment options beyond ten years, so management decisions should be made on a case-by-case basis.
Denosumab should be reviewed after three years, as per NICE recommendations, but the optimal duration of treatment is still under investigation[46]
. Some extension studies have demonstrated that benefits persist after eight to ten years of treatment[54],[55]
.
Special populations
Chronic kidney disease
Owing to the range of bone diseases that can present in patients with renal osteodystrophy (e.g. osteomalacia, adynamic bone disease and osteitis fibrosa cystica), management of osteoporosis requires a multidisciplinary team approach, and involving a nephrologist in care of patients who sustain fractures is paramount. It is important to ensure regular monitoring of serum-adjusted calcium, phosphate, parathyroid hormone (PTH) and vitamin D levels.
- Vitamin D supplementation— in patients with CKD stage 5, the mean prevalence of vitamin D insufficiency and deficiency has been shown to be as high as 82%[56]
. Therefore, monitoring of patients’ vitamin D levels and initiating active vitamin D supplementation in the form of alfacalcidol is essential. Beneficially, improving serum vitamin D levels decreases serum PTH level
[56]
; - Treatment of hyperphosphataemia— this condition is associated with reduced BMD and increased risk of fractures[56]
. Engaging dietitians to advise patients on the importance of a low-phosphate diet and the introduction of phosphate binders (i.e. sevelamer) can help in achieving normalisation of phosphate levels; - Treating secondary hyperparathyroidism— it has been shown that a persistently raised versus an isolated raised PTH level is associated with cortical bone loss[56]
. Cinacalcet increases the sensitivity of calcium receptors on the parathyroid gland thereby inhibiting the release of PTH and reducing resorption of demineralised bone in end-stage kidney disease (ESKD) patients (those with CKD stage 4 or 5 with creatinine clearance of <30mL/min).
At present, the use of bisphosphonates is contraindicated in patients with ESKD, owing to a lack of head-to-head clinical trials and the potential of bisphosphonates exacerbating low bone turnover and adynamic bone disease[56],[57]
.
Denosumab may be considered in patients with creatinine clearance <30mL/min. It has been reported to cause severe hypocalcaemia in patients with ESKD who require dialysis. The risk of significant hypocalcaemia is reduced by ensuring appropriate replenishment of the patient’s vitamin D levels[56],[57]
.
However, a nephrologist’s opinion should be sought prior to commencement of bone-protecting agents.
Parkinson’s disease
This is a neurodegenerative disorder typically affecting patients aged over 65 years[58]
. A study by Invernizzi et al. showed that at least 91% of female patients and 61% of male patients with Parkinson’s disease (PD) have either osteoporosis or osteopenia[59]
. Gait disturbances, low BMD, recurrent falls, postural instability, postural hypotension and polypharmacy all contribute to increased fracture risk in patients with PD[58]
.
Owing to the high incidence of osteoporosis in patients with PD, a holistic approach to the management of osteoporosis is required. This includes:
- Identifying and treating reversible factors — reducing the contributory effects of postural hypotension leading to falls and reducing muscle deconditioning via in-depth occupational therapy and physiotherapy assessment;
- Modifying patients’ lifestyle — providing dietary and smoking cessation advice;
- Ensuring correction of vitamin D and calcium levels;
- Recommending bone-protection agents upon confirmation of osteoporosis on DEXA scan or for high-risk individuals.
Dementia and cognitive impairment
Fragility fractures and dementia often co-exist in older people; the consensus is that dementia increases the risk of falls and fractures[4],[60]
. Patients with dementia have reduced dopamine activity, which causes a decline in motor function, leading to impairment of gait and balance[60]
. The use of medicines such as antipsychotics, sedatives and cholinesterase inhibitors used in Alzheimer’s disease can increase the risk of falls and fractures[61],[62]
. Patients with dementia have a higher prevalence of vitamin D deficiency and this should be addressed either via engaging a dietitian to improve their nutritional status or by providing vitamin D supplements[61],[63]
.
Bedridden patients
Healthy bones require mechanical stress to maintain their mass and strength; therefore, immobilisation for prolonged periods of time can result in bone atrophy and osteoporosis[64]
. Patients who are completely bedbound are at greatest risk of osteoporosis. Even ordinary forces encountered during wheelchair transfers, physical therapy activities or minor falls may cause fractures[65]
. BMD of the vertebral column decreases by around 1% per week of bed rest — nearly 50 times that of normal age-related bone loss[65]
. With bed rest, bones become spongy, which makes them easy to compress, deform or fracture[65]
.
Role of the pharmacist
A patient-centred approach can support informed adherence and pharmacists have a role in helping patients understand what osteoporosis is (including understanding that a fracture means a broken bone) and to reinforce the aims of treatment (i.e. to improve bone health and reduce fracture risk).
Concordance with oral bisphosphonates is poor, with reports that 50% of patients discontinue treatment after one year[66]
. The Royal Osteoporosis Society (ROS) has identified several challenges in this area, including:
- A lack of understanding of osteoporosis;
- The treatment aims not being fully explained;
- Patients’ fear of side effects;
- The idea that the treatment will not work;
- A lack of understanding in how to take the treatment correctly;
- A lack of visible signs that the treatment is working;
- The treatment being inconvenient or not suiting a patient’s lifestyle.
Pharmacists can help patients overcome some of these challenges by providing verbal or written instructions, and information about side effects; gathering support from family/carers; and referring the patient to the ROS helpline or local support groups. They can also provide essential lifestyle advice. Older patients who reside in nursing or residential homes should be offered the same support and carers should be properly informed.
Future perspective
There have not been many new drug developments in osteoporosis; however, in January 2018 the European Medicines Agency accepted a marketing authorisation application for a new drug called Evenityâ„¢ (romosozumab; Amgen). This drug has been in development for around 16 years and has a novel mechanism of action as it increases bone formation and reduces bone resorption simultaneously by inhibiting sclerostin to increase BMD and reduce the risk of fracture. At the time of writing, this drug is in phase III clinical trials. If approved, it has the potential to change the management of osteoporosis significantly.
There is also the possibility of pharmacists being integrated within local fracture liaison services (FLSs). These services aim to offer screening and treatment for osteoporosis, as well as care coordination, and educational and clinical governance support[3]
. An FLS is a proactive case-finding care pathway to ensure that patients who present with fragility fractures have a formal bone health assessment and targeted interventions that reduce subsequent fracture risk. Not all localities in the UK have an FLS, but considering demographic changes (with an increasing ageing population), an effective FLS has the potential to reduce the rate of increase of incident fractures.
References
[1] Kanis JA, Melton LJ 3rd, Christiansen C et al. The diagnosis of osteoporosis. J Bone Miner Res 1994;9(8):1137–1141. doi: 10.1002/jbmr.5650090802
[2] Johnell O & Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 2006;17(12):1726–1733. doi: 10.1007/s00198-006-0172-4
[3] National Osteoporosis Society. National quality standards for osteoporosis and prevention of fragility fractures. 2017. Available at: https://nos.org.uk/media/99099/op-standards.pdf (accessed April 2019)
[4] National Osteoporosis Guideline Group. Clinical guideline for the prevention and treatment of osteoporosis. 2018. Available at: https://www.sheffield.ac.uk/NOGG/NOGG%20Guideline%202017.pdf (accessed April 2019)
[5] van Staa TP, Dennison EM, Leufkens HG & Copper C. Epidemiology of fractures in England and Wales. Bone 2001;29(6):517–522. doi: 10.1016/S8756-3282(01)00614-7
[6] Scottish Intercollegiate Guidelines Network. SIGN 142: management of osteoporosis and the prevention of fragility fractures. 2015. Available at: https://www.sign.ac.uk/assets/sign142.pdf (accessed April 2019)
[7] Public Health England. Falls and fracture consensus statement: supporting commissioning for prevention. 2017. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/586382/falls_and_fractures_consensus_statement.pdf (accessed April 2019)
[8] Neuburger, J, Currie C, Wakeman R et al. The impact of a national clinician-led audit initiative on care and mortality after hip fracture in England: an external evaluation using time trends in non-audit data. Med Care 2015;53(8):686–691. doi: 10.1097/MLR.0000000000000383
[9] Svedbom A, Hernlund E, IvergaÌŠrd M et al.; EU Review Panel of IOF. Osteoporosis in the European Union: a compendium of country-specific reports. Arch Osteoporos 2013;8 :137. doi: 10.1007/s11657-013-0137-0
[10] Clunie G & Keen R. Osteoporosis. 2nd edn. Oxford: Oxford University Press; 2014
[11] Bilezikian JP. Osteoporosis in men. J Clin Endocrinol Metab 1999;84(10):3431–3434. doi: 10.1210/jcem.84.10.6060
[12] National Cancer Institute. NCI dictionary of cancer terms. 2004. Available at: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/bone-mineral-density (accessed April 2019)
[13] Goddard D & Kleerekoper M. The epidemiology of osteoporosis: practical implications for patient care. Postgrad Med 1998;104(4):54–72. doi: 10.3810/pgm.1998.10.441
[14] Watts NB. Adverse bone effects of medications used to treat non-skeletal disorders. Osteoporosis Int 2017;28(10):2741–2746. doi: 10.1007/s00198-017-4171-4
[15] World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. 1994. Available at: https://apps.who.int/iris/handle/10665/39142 (accessed April 2019)
[16] Christodoulou C & Cooper C. What is osteoporosis? Postgrad Med J 2003;79:133–138. Available at: https://pmj.bmj.com/content/postgradmedj/79/929/138.full.pdf (accessed April 2019)
[17] Sözen T, Özışık L & BaÅŸaran NÇ. An overview and management of osteoporosis. Eur J Rheumatol 2016;4(1):46–56. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5335887/pdf/ejr-4-1-46.pdf (accessed April 2019)
[18] National Institute for Health and Care Excellence. Osteoporosis: assessing the risk of fragility fracture. Clinical guideline [CG146]. 2017. Available at: https://www.nice.org.uk/guidance/cg146 (accessed April 2019)
[19] Kanis JA, Johnell O, Oden A et al. Smoking and fracture risk: a meta-analysis. Osteoporosis Int 2005;16(2):155–162. doi: 10.1007/s00198-004-1640-3
[20] Howe TE, Shea B, Dawson LJ et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev 2011;6(7). doi: 10.1002/14651858.CD000333.pub2
[21] National Osteoporosis Society. Strong, steady and straight: an expert consensus statement on physical activity and exercise for osteoporosis. 2018. Available at: https://nos.org.uk/strong-steady-and-straight/ (accessed April 2019)
[22] Tai V, Leung W, Grey A et al. Calcium intake and bone mineral density: systematic review and meta-analysis. BMJ 2015;351:h4183. doi: 10.1136/bmj.h4183
[23] Lewis JR, Radavelli-Bagatini S, Rejnmark L et al. The effects of calcium supplementation on verified coronary heart disease hospitalization and death in postmenopausal women: a collaborative meta-analysis of randomized controlled trials. J Bone Miner Res 2015;30(1):165–175. doi: 10.1002/jbmr.2311
[24] Candelas G, Martinez-Lopez JA, Rosario MP et al. Calcium supplementation and kidney stone risk in osteoporosis: a systematic literature review. Clin Exp Rheumatol 2012;30(6):954–961. PMID: 23137489
[25] Scientific Advisory Committee on Nutrition. Vitamin D and health. 2016. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/537616/SACN_Vitamin_D_and_Health_report.pdf (accessed April 2019)
[26] Martin TJ & Grill V. Bisphosphonates - mechanisms of action. Australian Prescriber 2000;23(6):130–132. doi: 10.18773/austprescr.2000.144
[27] Rodan AR & Reszka AA. Bisphosphonate mechanism of action. Curr Mol Med 2002;2(6):571–577. doi: 10.2174/1566524023362104
[28] Lin JH. Bisphosphonates: a review of their pharmacokinetic properties. Bone 1996;18(2):75–85. doi: 10.1016/8756-3282(95)00445-9
[29] eMC. Summary of product characteristics: Fosamax once weekly 70mg tablets. 2018. Available at: https://www.medicines.org.uk/emc/product/1281/smpc (accessed April 2019)
[30] eMC. Summary of product characteristics: Actonel once a week 35mg film-coated tablets. 2016. Available at: https://www.medicines.org.uk/emc/product/6757/smpc (accessed April 2019)
[31] eMC. Summary of product characteristics: Bonviva 150mg film-coated tablets. 2018. Available at: https://www.medicines.org.uk/emc/product/9383/smpc (accessed April 2019)
[32] Specialist Pharmacy Service. Do gastric adverse events influence choice of bisphosphonate for the treatment of osteoporosis? Specialist Pharmacy Service. 2018. Available at: https://www.sps.nhs.uk/articles/do-gastric-adverse-events-influence-the-choice-of-bisphosphonate-for-the-treatment-of-osteoporosis-2/ (accessed April 2019)
[33] Joint Formulary Committee. British National Formulary . 75th edn. London: Pharmaceutical Press; 2018
[34] eMC. Summary of product characteristics: Aclasta 5mg solution for infusion. 2018. Available at: https://www.medicines.org.uk/emc/product/210 (accessed April 2019)
[35] NHS Education for Scotland. Oral health management of patients at risk of medication-related osteonecrosis of the jaw: dental clinical guidance. 2017. Available at: http://www.sdcep.org.uk/wp-content/uploads/2017/04/SDCEP-Oral-Health-Management-of-Patients-at-Risk-of-MRONJ-Guidance-full.pdf (accessed April 2019)
[36] Medicines and Healthcare products Regulatory Agency. Bisphosphonates: very rare reports of osteonecrosis of the external auditory canal. 2015. Available at: https://www.gov.uk/drug-safety-update/bisphosphonates-very-rare-reports-of-osteonecrosis-of-the-external-auditory-canal (accessed April 2019)
[37] Medicines and Healthcare products Regulatory Agency. Bisphosphonates: use and safety. 2014. Available at: https://www.gov.uk/government/publications/bisphosphonates-use-and-safety/bisphosphonates-use-and-safety (accessed April 2019)
[38] National Institute for Health and Care Excellence. Osteoporosis. Quality standard [QS149]. 2017. Available at: www.nice.org.uk/guidance/qs149 (accessed April 2019)
[39] Medicines and Healthcare products Regulatory Agency. Bisphosphonates: atrial fibrillation. 2014. Available at: https://www.gov.uk/drug-safety-update/bisphosphonates-atrial-fibrillation (accessed April 2019)
[40] Medicines and Healthcare products Regulatory Agency. Bisphosphonates: osteonecrosis of the jaw. Medication safety alert. 2014. Available at: https://www.gov.uk/drug-safety-update/bisphosphonates-osteonecrosis-of-the-jaw (accessed April 2019)
[41] Medicines and Healthcare products Regulatory Agency. Denosumab: updated recommendations. 2014. Available at: https://www.gov.uk/drug-safety-update/denosumab-updated-recommendations (accessed April 2019)
[42] Medicines and Healthcare products Regulatory Agency. Denosumab, intravenous bisphosphonates: osteonecrosis of the jaw – further measures to minimise risk. 2015. Available at: https://www.gov.uk/drug-safety-update/denosumab-xgeva-prolia-intravenous-bisphosphonates-osteonecrosis-of-the-jaw-further-measures-to-minimise-risk (accessed April 2019)
[43] Medicines and Healthcare products Regulatory Agency. Bisphosphonates: atypical femoral fractures. 2014. Available at: https://www.gov.uk/drug-safety-update/bisphosphonates-atypical-femoral-fractures (accessed April 2019)
[44] Medicines and Healthcare products Regulatory Agency. Denosumab 60mg (Prolia): rare cases of atypical femoral fracture with long-term use. 2014. Available at: https://www.gov.uk/drug-safety-update/denosumab-60-mg-prolia (accessed April 2019)
[45] Medicines and Healthcare products Regulatory Agency. Denosumab: reports of osteonecrosis of the external auditory canal. 2017. Available at: https://www.gov.uk/drug-safety-update/denosumab-prolia-xgeva-reports-of-osteonecrosis-of-the-external-auditory-canal (accessed April 2019)
[46] National Institute for Health and Care Excellence. Denosumab for the prevention of osteoporotic fractures in postmenopausal women. Technology appraisal guidance [TA204]. 2010. Available at: www.nice.org.uk/guidance/ta204 (accessed April 2019)
[47] Langdahl BL, Teglbjærg CS, Ho PR et al. A 24-month study evaluating the efficacy and safety of denosumab for the treatment of men with low bone mineral density: results from the ADAMO trial. J Clin Endocrinol Metab 2015;100(4):1335–1342. doi: 10.1210/jc.2014-4079
[48] Bone HG, Bolognese MA, Yuen CK et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 2011;96(4):972–980. doi: 10.1210/jc.2010-1502
[49] National Institute for Health and Care Excellence. Raloxifene for the primary prevention of osteoporotic fragility fractures in postmenopausal women. Technology appraisal guidance [TA160]. 2018. Available at: https://www.nice.org.uk/guidance/ta160 (accessed April 2019)
[50] National Institute for Health and Care Excellence. Raloxifene and teriparatide for the secondary prevention of osteoporotic fragility fractures in postmenopausal women. Technology appraisal guidance [TA161]. 2018. Available at: https://www.nice.org.uk/guidance/ta161 (accessed April 2019)
[51] eMC. Summary of product characteristics: Evista 60mg film-coated tablets. 2016. Available at: https://www.medicines.org.uk/emc/product/3778/smpc (accessed April 2019)
[52] eMC. Summary of product characteristics: Forsteo 20 micrograms/80 microlitres solution for injection in pre-filled pen. 2017. Available at: https://www.medicines.org.uk/emc/product/2215/smpc (accessed April 2019)
[53] NHS England. Interim clinical commissioning policy statement: teriparatide for osteoporosis in men (adults). 2018. Available at: https://www.england.nhs.uk/wp-content/uploads/2018/07/1804-teriparatide.pdf (accessed April 2019)
[54] Papapoulos S, Lippuner K, Roux C et al. The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study. Osteoporosis International 2015;26(12):2773–2783. doi: 10.1007/s00198-015-3234-7
[55] Bone HG, Wagman RB, Brandi ML et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol 2017;5(7):513–523. doi: 10.1016/S2213-8587(17)30138-9
[56] Pimental A, Urena-Tores P, Zilikens MC et al. Fractures in patients with CKD- diagnosis, treatment, and prevention: a review by members of the European Calcified Tissue Society and the European Renal Association of Nephrology Dialysis and Transplantation. Kidney Int 2017;92(6):1343–1355. doi: 10.1016/j.kint.2017.07.021
[57] Kidney Disease Improving Global Outcomes. Clinical practice guideline update for the diagnosis, evaluation, prevention and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2017;7(1):1–59. doi: 10.1016/j.kisu.2017.04.001
[58] Torsney KM, Noyce AJ, Doherty KM et al. Bone health in Parkinson’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2014;85(10):1159–1166. doi: 10.1136/jnnp-2013-307307
[59] Invernizzi M, Carda S, Viscontini GS & Cisari C. Osteoporosis in Parkinson’s disease. Parkinsonism Relat Disord 2009;15(5):339–346. doi: 10.1016/j.parkreldis.2009.02.009
[60] Bäckman L, Lidenberger U, Li SC & Nyberg L. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: recent data and future avenues. Neurosci Biobehav Rev 2009;34(5);670–677. doi: 10.1016/j.neubiorev.2009.12.008
[61] Vun JSH, Ahmadi M, Panteli M et al. Dementia and fragility fractures: i ssues and solutions. Injury 2017;48:S10–S16. doi: 10.1016/j.injury.2017.08.031
[62] Gill SS, Anderson GM, Fischer HD et al. Syncope and its consequences in patients with dementia receiving cholinesterase inhibitors. A population-based cohort study. Arch Intern Med 2009;169(9):867–873. doi: 10.1001/archinternmed.2009.43
[63] Aspell N, Lawlor B & O’Sullivan M. Is there a role for vitamin D in supporting cognitive function as we age? Proc Nutr Soc 2018;77(2):124–134. doi: 10.1017/S0029665117004153
[64] Ehrlich PJ & Lanyon LE. Mechanical strain and bone cell function: a review. Osteoporosis Int 2002;13(9):688–700. doi: 10.1007/s001980200095
[65] Nigam Y, Knight J & Jones A. Effects of bed rest 3: musculoskeletal and immune systems, and skin. Nurs Times 2009;105(23):18–23. PMID: 19624052
[66] Sampalis JS, Adachi JD, Rampakakis E et al. Long-term impact of adherence to oral bisphosphonates on osteoporotic fracture incidence. J Bone Miner Res 2011;27(1):202–210. doi: 10.1002/jbmr.533