St Bartholomew's Hospital / Science Photo Library
Hospitalisation is known to increase the risk of developing venous thromboembolism (VTE) — a condition that most commonly includes deep vein thrombosis (DVT) or pulmonary embolism (PE). Hospital-acquired VTE, also known as hospital-associated or hospital-acquired thrombosis (HAT), causes a significant number of deaths and is estimated to cost the NHS £640m per year[1]
. HAT is the leading cause of preventable hospital mortality in the UK[2]
and includes any VTE that develops while a patient is in hospital or within 90 days of their discharge.
Background incidence of VTE is estimated to be 1–2 per 1,000 of the population[3]
and the incidence in the hospitalised patient population is considered to be around 3 per 1,000[4]
.
Several different factors are thought to increase the risk of developing VTE, some of which may be more likely to occur in the hospital setting. They are broadly categorised as prothrombotic changes to the blood, vascular wall injury and circulatory stasis (see Figure).
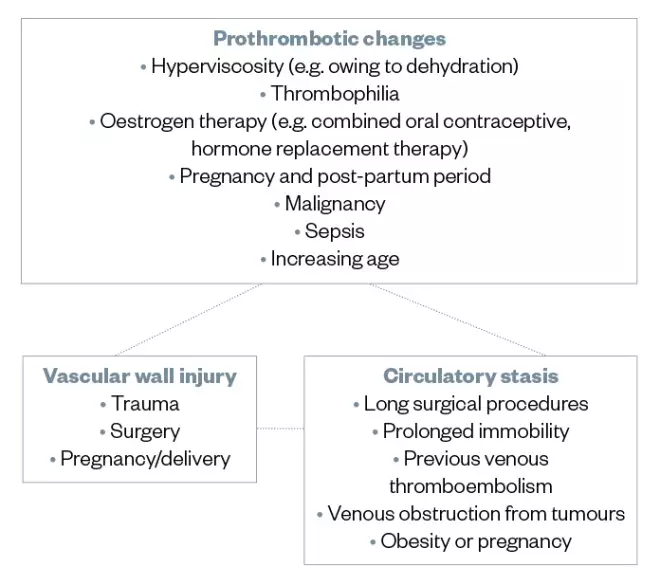
Figure: Examples of thrombosis risk factors that are thought to increase the risk of developing venous thromboembolism in the hospital setting, as per Virchow’s triad
Source: The Pharmaceutical Journal
In 2005, the House of Commons Health Select Committee published a report highlighting the significance of the HAT burden on morbidity and mortality in the UK[1]
. An expert working group on VTE prevention was formed in response, which published a proposed strategy to address the issue in 2007[5]
. A national VTE risk-assessment tool was published in 2008[6]
.
Early 2018 saw the publication of the National Institute for Health and Care Excellence (NICE) guideline NG89 — ‘Venous thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism’[7]
. NG89 was an update to and replacement of the NICE guideline CG92. There are several significant changes between the two, as highlighted in Table 1.
This article summarises some of the changes made regarding the use of pharmacological thromboprophylaxis (TP) and, where available, briefly discusses the evidence base from which the recommendations were made.
| Area | CG92 recommendation | NG89 recommendation | |
|---|---|---|---|
| Scope of guideline | The guideline applies to adults (defined as those aged 18 years and over) admitted to hospital. | The guideline applies to all inpatients aged over 16 years and those presenting to emergency departments with lower limb immobilisation. The guidance refers specifically to acute psychiatric inpatients; guidance for pregnant/ postpartum women has been expanded on. | |
| Risk-assessment tool | Healthcare professionals must use the risk criteria specified in the guideline. | Heathcare professionals may use any tool published by a UK body, professional network or peer-reviewed journal. | |
| Timing of first venous thromboembolism (VTE) risk assessment | This should be done on admission. | This should be done as soon as possible after admission or by the time of first consultant review. | |
| Timing of reassessment of VTE risk | This should be done within 24 hours of admission and whenever the clinical situation changes. | This should be done at the point of consultant review or when the clinical situation changes. For postpartum women, this should be done within six hours of delivery (including miscarriage and termination of pregnancy). | |
| Timing of first dose of pharmacological thromboprophylaxis (TP) | This should be done as soon as possible after risk assessment is complete. | This should be done as soon as possible and within 14 hours* of admission. | |
| Minimum recommended duration of prophylaxis | Prophylaxis should continue until the patient is no longer at an increased risk of VTE. | Prophylaxis should continue for a minimum of seven days for all medical and most surgical patients. | |
| Patients with renal impairment | The choice of pharmacological TP should be based on local policies and individual patient factors, including clinical condition (e.g. kidney failure). | Healthcare professionals should use low-molecular-weight heparin or unfractionated heparin if pharmacological prophylaxis is indicated. If needed, reduce the doses for patients with renal impairment, in accordance with locally agreed protocols. | |
| Specific recommendations for subgroups of patients | Medical | Guidance is given for the following patient groups: general medical patients, patients who have had a stroke, patients who have cancer, patients receiving palliative care and patients with central venous catheters (CVCs) in situ. | Guidance has been expanded to include acute coronary syndrome. The term “general” was replaced with “acutely ill” medical patients, and the term “stroke” was redefined as “acute stroke”. Changes to recommendations were made for patients with certain types of cancer. Reference to CVCs were removed. |
| Surgical (non-orthopaedic) | Guidance is given for the following patient groups: patients undergoing cardiac, gastrointestinal, gynaecological, thoracic, urological and neurological (cranial or spinal) surgery. | Guidance has been expanded to include patients undergoing thoracic, vascular, oral and maxillofacial and ear, nose and throat surgery. | |
| Surgical (orthopaedic) | Guidance is given for the following patient groups: patients who have had major trauma or spinal injuries, patients who are receiving critical care, and patients who are pregnant or up to six weeks postpartum. | Guidance has been expanded to include patients with fragility fractures of the pelvis and proximal femur; patients undergoing nonarthroplasty orthopaedic knee surgery; and foot and ankle, and upper limb patients. The term “lower-limb casts” was replaced with “lower-limb immobilisation”. | |
| Other | Guidance is given for the following patient groups: patients who have had major trauma or spinal injuries, patients who are receiving critical care, and patients who are pregnant or up to six weeks postpartum. | “Postpartum” is now defined as referring to women who have had a baby, a miscarriage or a termination of pregnancy. | |
*This is in line with the current NHS policy that states that all emergency admissions must be seen and have a thorough clinical assessment by a consultant within 14 hours of admission to hospital. | |||
Assessing and reassessing the risk of venous thromboembolism
The updated NICE guideline recommends that all patients and pregnant women, women who have given birth, or who had a miscarriage or termination within the past six weeks, are risk assessed as soon as possible after admission to hospital or at the time of the first consultant review[7]
.
Unlike in the previous guideline, no risk-assessment criteria are stipulated in NG89. The NICE guideline committee reviewed evidence for several VTE risk-assessment tools and agreed there is a lack of good-quality evidence for any single tool, particularly in medical patients (with no evidence found to recommend a specific tool to use for pregnant women). While they acknowledged that neither the national tool nor the tool developed by the Royal College of Obstetricians and Gynaecologists (RCOG)[8]
have been validated, and as there is concern that both lead to overprescribing of prophylaxis in certain patients, they recognised that these are the most widely used risk-assessment tools in the UK. Furthermore, no evidence was found for the effectiveness of any VTE risk tool specifically for reassessment. Therefore, the committee did not make any recommendation about the use of a specific tool for assessment or reassessment of risk.
The guideline recommends that patient risk should be reassessed at the time of senior review for medical, surgical and trauma patients; this was considered an efficient use of resources as it could be included in the review. Women who have just given birth, or had a miscarriage or termination, should be reassessed within six hours to allow time to check outcomes and the change in bleeding risk from the prelabour state.
For all patients, VTE and bleeding risk should be balanced against each other at each assessment when deciding whether or not to offer pharmacological TP.
Timing of the first dose of pharmacological thromboprophylaxis
The NICE committee noted that many patients admitted to hospital — especially emergency admissions — do not receive a dose of pharmacological TP on the day of their admission, owing to the fact that they are admitted after the set daily time of administration. The new recommendation that patients should receive their first dose of pharmacological TP within 14 hours of admission aims to address this issue.
Doses of pharmacological thromboprophylaxis in patients with renal impairment
The updated NICE guideline includes new advice regarding patients with renal impairment and gives healthcare professionals the option to use low-molecular-weight heparin (LMWH) or unfractionated heparin (UFH) at reduced doses[7]
. The decision about which option should be used, however, should be based on multidisciplinary or senior opinion, or locally agreed protocols. The guideline does not make further specific recommendations regarding doses and does not discuss any evidence for this recommendation (see Useful resources).
Under 18s and drug licences
None of the pharmacological TP recommended in the guideline are licensed for use in patients who are aged under 18 years. Therefore, for patients aged 16–18 years, these agents should be used “off-label”.
Interventions for pregnant women and women up to six weeks postpartum
There were four studies included in the NICE review of the evidence, and the pharmacological interventions identified within them were LMWH and UFH. The evidence was limited to women who had delivered by caesarean section and was not considered to be of good quality.
The committee concluded UFH should not be recommended, owing to lack of evidence of efficacy and its short half-life, which means users require multiple daily injections. The increased risk of osteoÂporosis with UFH was also considered.
The evidence for using LMWH was found to be limited; however, it did note a possible clinical benefit for its use in reducing DVT. There was no clinical difference identified between standard- and extended-duration TP; as a result, the committee advised a seven-day duration, as this is the average duration in the trials presented throughout the guideline. This is contrary to the advice in the RCOG guideline, which recommends using LMWH for a minimum of ten days after delivery in women for whom it is indicated.
The advice regarding withholding LMWH for women who are in active labour allows for the safe administration of epidurals and minimises the risk of bleeding.
The committee also noted that there is no evidence for the use of weight-adjusted dosing in this population, despite the RCOG guideline recommending it. Therefore, they did not recommend this practice in the updated guideline.
Interventions for medical patients
The advice summarised below regarding pharmacological TP assumes that thrombosis risks have been deemed to outweigh bleeding risks, and that no contraindication to pharmacological TP exists.
Acutely ill medical patients
NG89 recommends offering acutely ill medical patients LMWH first-line, and fondaparinux sodium if LMWH is contraindicated[7]
— the previous guideline, CG92, recommended either with an equal weighting. After review of the evidence, which included ten new studies since CG92’s final update, the committee considered the clinically beneficial effects of LMWH and fondaparinux sufficient to adopt the CG92’s recommendation; however, as fondaparinux use is not cost effective, it is only recommended when LMWH is contraindicated.
Direct oral anticoagulants were evaluated as offering equal benefit in the reduction of VTE risk, compared to LMWH, but with an increased risk of major bleeding. Contrary to CG92’s advice to continue pharmacological prophylaxis until the patient is no longer at increased risk of VTE, NG89 advises the administration of direct oral anticoagulants for a minimum of seven days in this population[7]
. While it is not made explicit in the update why the advice has changed, two of the papers referenced appear to explain the recommendation.
The MEDENOX study[9]
and the PREVENT study[10]
gave prophylactic LMWH or placebo to patients aged over 40 years who had several acute or chronic medical conditions for between 6 days and 14 days (a median length of stay of around 7 days). Both studies demonstrated a significant reduction in the risk of developing VTE in patients with TP compared with placebo (63% and 49% in each study, respectively). However, it is not clear whether the guideline recommends that medical patients receive TP once they have left hospital if their admission is fewer than seven days in duration. Current practice does not normally provide medical patients with extended (beyond discharge) prophylaxis.
Patients with cancer
The updated NICE guideline recommends that healthcare professionals consider using pharmacological TP for patients with myeloma who are receiving chemotherapy with thalidomide, pomalidomide or lenalidomide with steroids, choosing either aspirin (75mg or 150mg) or LMWH[7]
.
The committee reviewed three randomised controlled trials (RCTs) for this population; two looked specifically at the use of prophylactic LMWH and aspirin (100mg daily) to reduce the risk of VTE in patients with previously untreated myeloma, who were receiving treatment with thalidomide or lenalidomide. Palumbo et al. found LMWH and aspirin showed similar efficacy in reducing serious thromboembolic events in patients who received thalidomide-containing regimens[11]
. Larocca et al. concluded that aspirin could be an effective and less expensive alternative to LMWH in patients with a low thromboÂembolic risk receiving lenalidomide[12]
, and Chalayer et al. suggested that aspirin is more cost effective[13]
. However, the advice in NG89 differs from that given by the BÂritish Society of Haematology, which recommends aspirin for patients who are at low risk of developing VTE and LMWH for those at high risk[14]
.
The NICE committee advises that TP with LMWH should be considered for patients with pancreatic cancer who are receiving chemotherapy and is continued for the duration of their treatment, as rates of thromboembolism are high in this population. The CONKO-004 trial demonstrated that the cumulative incidence rates of symptomatic VTEs were higher in the observation group than the LMWH group[15]
. However, owing to the uncertainty of the evidence base in both these populations, the guideline makes a weak recommendation (as the word “consider” has been used).
Apart from in these populations, the guideline advises not to offer TP to patients with cancer who are receiving disease-modifying treatments and who are mobile, unless they are also at increased risk of VTE owing to another factor. In much of the evidence reviewed, there was no clinically important difference in outcome between the cancer patients who received prophylaxis and those who did not.
Patients receiving palliative care
As in the CG92 NICE guideline, NG89 says consideration should be given to using pharmacological TP for patients receiving palliative care, taking into account likely life expectancy and the views of the patient/carers. The thrombosis and bleeding risks, which should be reviewed daily, should also be considered when making a decision. There were no relevant clinical studies identified on review; therefore, the committee extrapolated data from the acutely ill medical patient population and offered the same recommendation. Expanding on previous advice, the updated guideline recommends that patients in the last days of life should not be offered TP because there is little health-economic or patient quality-of-life benefit in doing so[7]
.
Patients admitted to critical care
Recommendations for this population are extended in the updated NICE guidelines, stating that TP with LMWH should be provided as long as it is not contraindicated. The committee reviewed one RCT for pharmacological TP, which evaluated the use of dalteparin versus UFH[16]
. The study showed the proportion of patients with PE was significantly lower, and there was a clinically important difference in mortality rate in the dalteparin group compared with the UFH group. The recommendation to consider mechanical TP, such as elastic compression stockings, if pharmacological TP is contraindicated, is owing to the high risk of VTE in this population.
Patients with psychiatric illness
People in this group who are admitted to hospital may be at increased risk of developing VTE when acutely unwell owing to several factors, including the use of antiÂpsychotic medicine. No relevant clinical studies were identified for this population; therefore, the NICE committee made recommendations in line with those for the acutely ill medical patients. However, the committee advises that TP is continued until the patient is no longer at increased risk of VTE, rather than for a minimum of seven days.
Interventions for patients having surgery
Lower-limb immobilisation
This term is used in the updated guideline to replace “lower-limb plaster casts”. The new recommendation is to consider pharmacological TP with LMWH or fondaparinux (previously LMWH or UFH) and to consider stopping TP if the period of immobilisation continues beyond 42 days. The committee reviewed ten studies and concluded that both LMWH and fondaparinux provided a clinically important reduction in the risk of developing DVT when compared with no prophylaxis. Two of these studies showed a benefit for fondaparinux over LMWH[17],[18]
, but the committee felt that the overall evidence did not support one agent over the other; therefore, they recommended that either may be used. The updated guideline also advises to consider stopping prophylaxis if lower-limb immobilisation continues beyond 42 days — not until cast removal, as was recommended in the previous version — as this is the information provided within the trials reviewed.
Fragility fracture of the pelvis, hip and proximal femur
This patient group has been expanded to contain more fracture types in the updated NICE guideline. It now recommends that patients receive LMWH or fondaparinux for one month post-operatively, as well as pre-operatively if surgery is delayed beyond the day after admission. The evidence reviewed for the updated guideline included nine new studies; of note in this review, the PEP trial[19]
was included (this was excluded from the previous version owing to concerns about its methodology).
The PEP trial evaluated the use of aspirin as TP in this patient population. More than 50% of the patients included were also receiving either LMWH or UFH, and around 30% were using anti-embolism stockings (AES); however, the overlap of patients, if any, is not known. The trial included aspirin when combined with other prophylaxis, but not aspirin alone. The bleeding outcomes were not adequately reported so were excluded from the NG89 review. The bleeding effect of using aspirin for TP is still unknown.
Elective hip and knee replacement
The updated NICE guideline recommends that patients who have had hip arthroplasty are offered VTE prophylaxis of: LMWH for 10 days followed by aspirin (75mg or 150mg) for a further 28 days; or LMWH for 28 days (with AES until discharge); or rivaroxaban (within its marketing authorisation). Use of apixaban or dabigatran can be considered if none of the other options are suitable.
The committee reviewed a large amount of clinical evidence for both patient groups. Nine new studies in hip arthroplasty were included in the update; however, the evidence for the recommendation of aspirin use after a short course of LMWH appears to be based on only one study. In this study, EPCAT I, patients who underwent elective hip arthroplasty (mean age of around 57 years) received 10 days of prophylactic dalteparin, and then 28 days of either dalteparin or aspirin (81mg orally)[20]
. Aspirin was non-inferior at preventing VTE, but the trial was stopped early because of slow enrolment.
LMWH for 10 days plus aspirin for 28 days was the most cost-effective option, as well as the top-ranked intervention for the clinical outcomes of PE and major bleeding. As this recommendation is based on a single trial and there are small differences in cost-effectiveness among the included interventions, the committee opted to give a choice of options rather than recommending only one.
For knee arthroplasty patients, the recommendation in the updated guideline is to offer either: LMWH (with AES until discharge), aspirin alone (75mg or 150mg) or rivaroxaban (within its mÂarketing authorisation) for 14 days post-operatively. Use of apixaban or dabigatran can be considered if these initial options are not suitable. An additional 14 new studies in knee arthroplasty were included but, again, the evidence for recommending aspirin is based on only one trial. Zou et al. compared prophyÂlactic rivaroxaban, enoxaparin and aspirin 100mg, which were commenced 12 hours after surgery and continued for 14 days[21]
. There were no significant differences in the incidence of DVT between the enoxaparin group and the aspirin group; the incidence of DVT was lower in the rivaroxaban group, but this was associated with more hidden blood loss and wound complications.
Non-arthroplasty orthopaedic knee surgery
Some five studies were reviewed for this population, all of which involved arthroscopy procedures. One study looked at patients undergoing major arthroscopy (mean combined surgery and anaesthetic time of over one hour)[22]
; one looked at minor arthroscopy (less than one hour)[23]
; however, the times were not reported in the other three studies.
Despite only identifying evidence for the arthroscopy population, the recommendation applies to all types of non-arthroplastÂy. The committee concluded that the evidence reviewed was of poor quality. Furthermore, the NICE orthopaedic subgroup committee advised that the risk of VTE is minimal if the anaesthesia time is lower than 90 minutes and the patient is at low risk of VTE, otherwise prophylaxis with LMWH should be considered on an individual basis. The recommended 14-day duration of LMWH was extrapolated from the knee arthroplasty population.
Elective spinal surgery
Just one RCT for the use of pharmacological TP in patients undergoing elective spinal surgery was included in the review. Du et al. compared prophylactic rivaroxaban with parnaparin[24]
. Both were given 6–8 hours after surgery and were continued for 14 days. Rivaroxaban was as effective as parnaparin against VTE, without increasing the risk of postÂ-operative bleeding. However, the evidence was graded very low by the committee. Since rivaroxaban does not have a license for use in spinal surgery and LMWH is currently used in standard practice, the latter was recommended.
The start time for administering post-surgery pharmacological TP was discussed. Considering patient and surgical factors, this should begin 24–48 hours post-operatively and should be continued for 30 days. The committee felt that senior healthcare professionals who are able to accurately identify if the patient has a low bleeding risk should be involved in any decisions to start pharmacological TP earlier than this.
Spinal injury
The review included four studies in this group, but the majority of the evidence was deemed to be of very low quality. Patients with a spinal injury are at high risk of VTE but the consequence of bleeding can be catastrophic. The committee agreed that pharmacological TP with LMWH can start 24 hours after the spinal injury when the bleeding risk has reduced, providing there are no immediate plans to operate. The duration of use is at the discretion of the healthcare professional.
Cranial surgery
Some five RCTs in this group were included in the NICE review; four looked at patients with intracranial tumour and one was non-tumour specific. Much of the evidence was of lower quality as the studies were small and likely to be underpowered. The committee generally supported the recommendations made in CG92 to give LMWH 24–48 hours post-operatively (and pre-operatively no less than 24 hours before surgery), but felt “consider” was a more appropriate recommendation given the uncertainty of the evidence.
Major trauma
This population is at high risk of both VTE and bleeding, and there were high VTE rates in the ten studies reviewed, leading to the recommendation regarding mechanical prophylaxis. Of the studies that looked at pharmacological TP only, the possible benefits of UFH versus no prophylaxis were only observed in one study[25]
.
When UFH was compared with LMWH, there were fewer incidences of proximal vein thrombosis in the LMWH group, but the rate of bleeding was higher[26]
. The NICE committee considered that there was insufficient evidence to recommend a specific pharmacological agent in this population. Nevertheless, they recommend considering the use of pharmacological TP for patients with serious or major trauma as soon as possible when the risk of VTE outweighs the risk of bleeding.
Abdominal (including bariatric) surgery
The committee reviewed 67 studies for the abdominal surgery population, 5 of which were new. Despite the quantity of evidence reviewed, the majority of it was cÂonsidered to be of moderate-to-very-low quality. They noted there was evidence that LMWH and fondaparinux were better than no prophylaxis, but not whether one was better than the other.
Combination strategies with mechanical methods appear to be the most effective at reducing the risk of DVT; therefore, the updated NICE guideline recommends pharmacological TP with either LMWH or fondaparinux to be used along with mechanical methods for a minimum of seven days[7]
. This should be extended to 28 days post-operatively for patients who have had major cancer surgery in the abdomen.
Bariatric surgery was considered separately to other types of abdominal surgery as these patients already have at least one risk factor for VTE. As a result, the guideline recommends these patients only receive pharmacological TP if thrombosis risk outweighs bleeding risk. Just one of the three studies that were reviewed used a LMWH that is licensed in the UK. Steele et al. observed that both enoxaparin and fondaparinux were better than no prophylaxis, but there was insufficient evidence to suggest that one was better than the other[27]
. The committee considered the recommendations for abdominal surgery to be applied to bariatric surgery, since the former is a subset of the latter.
The seven-day recommendation in this group reflects the average duration of trials; the committee agreed this should be extended to 28 days for cancer surgery because the evidence identified was for this duration.
Cardiac surgery
This population may be at increased risk of both VTE (owing to generally increased age and long anaesthesia time) and bleeding (owing to use of antiplatelet medicine and possible intra-operative use of heparin). Only three studies were reviewed for this population, which the committee acknowledged was a small amount of evidence. Therefore, they considered that similar recommendations for pharmacological TP could be made for this population as for abdominal surgery; namely that patients who are undergoing cardiac surgery and are not having other antiÂcoagulation therapy receive either LMWH (first-line) or fondaparinux for a minimum of seven days. While there is some evidence for the use of fondaparinux, it does not have a licence for this indication, so the committee recommended using it only if LMWH is contraindicated.
Vascular surgery
This population may also be at increased risk of both VTE and bleeding. The committee found very little RCT evidence in the open vascular surgery population, but thought it was appropriate to recommend the use of LMWH for a minimum of seven days for those at low risk of bleeding, owing to their likelihood of extended immobility. There was little direct evidence for the amputation population, but these patients are known to have a very high VTE risk and, in view of this, the committee felt it was likely that these patients would need pharmacological TP (but did not specify which agent to use).
Despite more varicose vein surgery being carried out under local anaesthetic using minimally invasive techniques, the risk of post-procedure VTE is such that the committee suggests pharmacological TP with LMWH to be considered for at-risk patients for a minimum of seven days. The seven-day recommendation in the vascular surgery group is extrapolated from the abdominal surgery trials.
Other surgical subgroups
The NICE committee could not locate any relevant clinical studies for some surgical populations. In these instances, the committee considered advice from other sources and made recommendations based on this (see Table 2).
| Surgical subgroup | Recommendation made | Basis of recommendation |
|---|---|---|
| Foot and ankle orthopaedic surgery | Consider pharmacological thromboprophylaxis (TP) in any of the following circumstances:
| The advice from the orthopaedic subgroup. |
| Upper-limb orthopaedic surgery | Consider pharmacological TP in any of the following circumstances*:
| The committee’s discussion†. |
| Thoracic surgery | Consider pharmacological TP (low-molecular-weight heparin [LMWH] first-line; fondaparinux second-line) for a minimum of seven days where VTE risk outweighs bleeding risk. | The recommendation is extrapolated from evidence for the abdominal surgery population‡. |
| Head and neck surgery (oral and maxillofacial or ear, nose and throat) | Consider pharmacological TP with LMWH for a minimum of seven days where VTE risk outweighs bleeding risk. | The recommendation is extrapolated from evidence for the abdominal surgery population. |
| * Where patients use their arms to support mobilisation. †VTE risk is minimal, especially in short procedures where people use their arms to support mobilisation and arm surgery would adversely affect mobility. ‡ Fondaparinux is not licensed in this population. | ||
Role of the pharmacist
Pharmacists will be involved in a variety of ways when implementing the update NICE guidelines (see Box).
Box: How the pharmacy team can support the delivery of effective pharmacological thromboprophylaxis
Pharmacists may perform many functions that support the provision of timely and appropriate thromboprophylaxis (TP), such as:
- Playing a strategic role:
- By updating local guidelines in accordance with national recommendations;
- By liaising with various specialty clinicians to ensure a harmonised approach to care;
- Critically appraising the different venous thromboembolism (VTE) risk-assessment tools available;
- Informing the hospital’s thrombosis committee, or relevant parties, of certain medicines’ advantages and disadvantages for different groups of patients;
- Developing and implementing prompts that support the prescribing of TP where appropriate;
- Preparing patient information leaflets on agents used for TP;
- Investigating and appraising the evidence for the off-label use of pharmacological agents in certain patient groups (e.g. people aged under 18 years);
- Leading on the implementation of research recommendations given in the National Institute for Health and Care Excellence’s NG89 guideline, such as developing dose strategies for obese patients;
- Monitoring the relevant clinical parameters for patients who are on TP and check that the appropriate dose has been prescribed for that patient based on their weight and kidney function;
- Ensuring ward stock lists are appropriate and stock levels are adequate (this can be performed by pharmacists or supported by other pharmacy staff).
Ward-based pharmacy staff also play an important role by:
- Ensuring patients receive timely and appropriate TP;
- Ensuring the completion of the risk assessment and, if appropriate, the prescribing of TP;
- Being actively involved in supporting ward audits to ensure risk assessment, prescribing and administration of prophylaxis is being undertaken;
- Patient counselling regarding use of TP and employing general measures such as mobilising, keeping well hydrated and recognising the signs and symptoms of VTE (this can be undertaken by the pharmacist or supported by a suitably trained medicines management technician).
Furthermore, independent prescribing pharmacists may themselves undertake the role of prescribing TP in certain areas, such as pre-operative assessment clinics.
Summary
Undoubtedly, many patients who are admitted to hospital are at increased risk of developing VTE. Identifying at-risk patients is imperative and, over the past decade and a half, much work has been done to embed this risk-assessment process into standard patient care. Risk assessment is only one element of the VTE-prevention strategy, however, and will only prove effective if at-risk patients are offered appropriate intervention(s) in a timely manner.
There are many areas of further development needed in this field, for example the validation of the national tool and developing dosing strategies for patients at extremes of bodyweights and in patients aged under 18 years. Pharmacy staff of various grades and disciplines can get involved in supporting the implementation of thrombosis-prevention strategies within their clinical areas, and help deliver effective, patient-centred care in order to reduce the number of patients who develop hospital-acquired thrombosis.
Useful resources
- Thrombosis UK: https://www.thrombosisuk.org/
- UKMi Medicines Q&A: Should prophylactic doses of low molecular weight heparins be used in patients with renal impairment? Available at: https://www.sps.nhs.uk/articles/should-prophylactic-doses-of-low-molecular-weight-heparins-be-used-in-patients-with-renal-impairment/
- NHS England: The VTE prevention e-learning course. Available at: https://www.e-lfh.org.uk/programmes/venous-thromboembolism-public-access/
References
[1] House of Commons Health Committee. The prevention of venous thromboembolism in hospitalised patients. 2005. Available at: https://publications.parliament.uk/pa/cm200405/cmselect/cmhealth/99/99.pdf (accessed February 2019)
[2] Thrombosis UK. Thrombosis statistics. 2018. Available at: https://www.thrombosisuk.org/thrombosis-statistics.php (accessed February 2019)
[3] White RH. The epidemiology of venous thromboembolism. Circulation 2003;107(23 Suppl 1):I1–I4. doi: 10.1161/01.CIR.0000078468.11849.66
[4] Heit JA. Estimating the incidence of symptomatic postoperative venous thromboembolism: the importance of perspective. JAMA 2012;307(3):306–307. doi: 10.1001/jama.2011.2013
[5] Department of Health. Report of the independent expert working group on the prevention of venous thromboembolism in hospitalised patients. 2007. Available at: http://www.venous-thromboembolism.org/reports/DH_073950.pdf (accessed February 2019)
[6] Department of Health. Risk assessment for venous thromboembolism. 2010. Available at: http://webarchive.nationalarchives.gov.uk/20130123195034/http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_088215 (accessed February 2019)
[7] National Institute for Health and Care Excellence. Venous thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism. NICE guideline [NG89]. 2018. Available at: https://www.nice.org.uk/guidance/ng89 (accessed February 2019)
[8] Royal College of Obstetricians & Gynaecologists. Reducing the risk of venous thromboembolism during pregnancy and the puerperium. Green-top guideline No. 37a. 2015. Available at: https://www.rcog.org.uk/globalassets/documents/guidelines/gtg-37a.pdf (accessed February 2019)
[9] Samama MM, Cohen AT, Darmon JY et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. N Engl J Med 1999;341(11):793–800. doi: 10.1056/NEJM199909093411103
[10] Leizorovicz A, Cohen AT, Turpie AG et al. Randomised, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients.Circulation 2004;110(7):877–879. doi: 10.1161/01.CIR.0000138928.83266.24
[11] Palumbo A, Cavo M, Bringhen S et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a phase III, open-label, randomized trial. J Clin Oncol 2011;29(8):986–993. doi: 10.1200/JCO.2010.31.6844
[12] Larocca A, Cavallo F, Bringhen S et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood 2012;119(4):933–939. doi: 10.1182/blood-2011-03-344333
[13] Chalayer E, Bourmaud A, Tinquaut F et al. Cost-effectiveness analysis of low-molecular-weight heparin versus aspirin thromboprophylaxis in patients newly diagnosed with multiple myeloma. Thrombosis Research 2016;145:119–125. doi: 10.1016/j.thromres.2016.08.008
[14] Watson HG, Keeling DM, Laffan M et al. British Committee for Standards in Haematology. Guideline on aspects of cancer-related venous thrombosis. British J Haem 2015;170(5):640–648. doi: 10.1111/bjh.13556
[15] Pelzer U, Opitz B, Deutschinoff G et al. Efficacy of prophylactic low-molecular weight heparin for ambulatory patients with advanced pancreatic cancer: outcomes from the CONKO-004 trial. J Clin Oncol 2015;33(18):2028–2034. doi: 10.1200/JCO.2014.55.1481
[16] Cook D, Meade M, Guyatt G et al. Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med 2011;364(14):1305–1314. doi: 10.1056/NEJMoa1014475
[17] Bruntink MM, Groutars YME, Schipper IB et al. Nadroparin or fondaparinux versus no thromboprophylaxis in patients immobilised in a below-knee plaster cast (PROTECT): a randomised controlled trial. Injury 2017;48(4):936–940. doi: 10.1016/j.injury.2017.02.018
[18] Samama CM, Lecoules N, Kierzek G et al. Comparison of fondaparinux with low molecular weight heparin for venous thromboembolism prevention in patients requiring rigid or semi-rigid immobilization for isolated non-surgical below-knee injury. J Thromb Haemost 2013;11(10):1833–1843. doi: 10.1111/jth.12395
[19] Pulmonary Embolism Prevention (PEP) trial Collaborative Group. Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet 2000;355(9212):1295–1302. doi: 10.1016/S0140-6736(00)02110-3
[20] Anderson DR, Dunbar MJ, Bohm ER et al. Aspirin versus low-molecular-weight heparin for extended venous thromboembolism prophylaxis after total hip arthroplasty: a randomized trial. Ann Intern Med 2013;158(11):800–806. doi: 10.7326/0003-4819-158-11-201306040-00004
[21] Zou Y, Tian S, Wang Y & Sun K. Administering aspirin, rivaroxaban and low-molecular-weight heparin to prevent deep venous thrombosis after total knee arthroplasty. Blood Coagul Fibrinolysis 2014;25(7):660–664. doi: 10.1097/MBC.0000000000000121
[22] Wirth T, Schneider B, Misselwitz F et al. Prevention of venous thromboembolism after knee arthroscopy with low-molecular weight heparin (reviparin): results of a randomized controlled trial. Arthroscopy 2001;17(4):393–399. doi: 10.1053/jars.2001.21247
[23] van Adrichem RA, Nemeth B, Algra A et al. Thromboprophylaxis after knee arthroscopy and lower-leg casting. N Engl J Med 2017;376(6):515-525. doi: 10.1056/NEJMoa1613303
[24] Du W, Zhao C, Wang J et al. Comparison of rivaroxaban and parnaparin for preventing venous thromboembolism after lumbar spine surgery. J Orthop Surg Res 2015;10:78. doi: 10.1186/s13018-015-0223-7
[25] Knudson MM, Lewis FR, Clinton A et al. Prevention of venous thromboembolism in trauma patients. J Trauma 1994;37(3):480–487. doi: 10.1097/00005373-199409000-00025
[26] Geerts WH, Jay RM, Code KI et al. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. N Engl J Med 1996; 335(10):701–707. doi: 10.1056/NEJM199609053351003
[27] Steele KE, Canner J, Prokopowicz G et al. The EFFORT trial: preoperative enoxaparin versus postoperative fondaparinux for thromboprophylaxis in bariatric surgical patients: a randomized double-blind pilot trial. Surg Obes Relat Dis 2015;11(3):672–683. doi: 10.1016/j.soard.2014.10.003