James King-Holmes / Science Photo Library
In this article you will learn:
- The approaches used to correctly diagnose epilepsy in children
- How to select appropriate drug treatments for children with epilepsy
- Potential future therapeutic options for the management of epilepsy
The epilepsies are the most common and chronic neurological disorders of childhood. The prevalence of epilepsy is approximately one in every 240 children, which equates to around 51,000 of the 12 million children in the UK[1],
[2]
; this is likely to be an underestimate and is probably closer to 60,000. The incidence of epilepsy in children is reported to range from 41 to 187 per 100,000; the higher incidence is seen in underdeveloped countries, particularly in rural areas[3]
.
It has been suggested that the incidence (and eventually the prevalence) since the 1990s has fallen, possibly on account of improved diagnosis and reduced risk factors[4]
. However, the data should be viewed with caution, as they were gathered in primary care (general practice) on the basis of repeat prescriptions of an antiepileptic drug (AED)[4]
. This precludes a significant minority of children with epilepsy that may not require treatment with an AED, or where parents have declined drug treatment for their child. Finally, misdiagnosis of epilepsy in children ranges from 10–30%, depending on the population studied, complicating the identification of accurate rates of incidence and prevalence of paediatric epilepsies[5],
[6]
.
The Classification and Terminology Committee of the International League Against Epilepsy (ILAE) is responsible for the regular review and update of the classification of seizures and epilepsies, including epilepsy syndromes. The classification has continued to be revised since its inception in 1960[7],[8],[9],[10],[11]
, with the most recent classification of 2010[11]
described as a ‘work in progress’, which has not been universally accepted. A detailed and critical commentary on the current classification is outside the remit of this article but can be found elsewhere[12]
.
Diagnosis
The management of epilepsy, including specific AED treatment, must begin with establishing a correct diagnosis on four different levels:
- Recognition of the paroxysmal episodes (‘seizures’) being epileptic in origin and not caused by another non-epileptic paroxysmal disorder (there are many different paroxysmal episodes that occur in children and that are often misdiagnosed as epilepsy).
- Classification of the seizure type (or types) using the ILAE Classification (see ‘2010 ILAE Classification of seizure types’).
- Identification of the epilepsy syndrome (using the same ILAE Classification).
- Identification of any underlying cause.
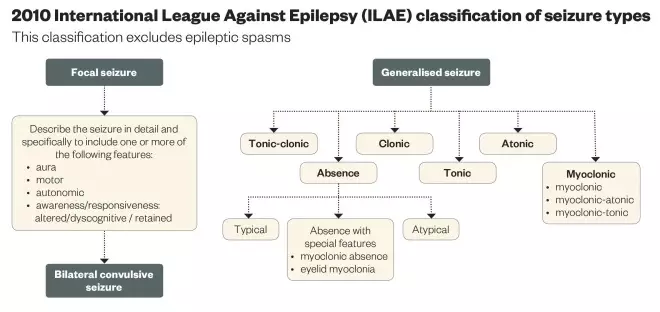
The syndromic approach provides useful information on the most appropriate AED to prescribe (and which AEDs to not prescribe), the likely long-term outcome and, to a lesser extent, possible recurrence in any siblings. The outcomes include: the likelihood of obtaining seizure-freedom with antiepileptic medication; the epilepsy showing spontaneous (terminal) remission; and the child’s developmental (learning) and social potential.
The identification of an epilepsy syndrome is based on the following criteria:
- Seizure type (or types) that the child is experiencing;
- Age at onset of the seizures;
- The child’s development/cognitive profile;
- Results of an electroencephalogram (EEG); ideally this should be both ictal (the EEG recorded during a seizure) and inter-ictal (the background EEG recorded between seizures).
The presence or absence of a family history of epilepsy is also considered by some to be an additional, but less important, clinical criterion.
In certain situations, additional investigations (specifically neuroimaging with magnetic resonance imaging [MRI] and genetic analysis [micro-array and DNA analyses]) will also be indicated to help identify both the epilepsy syndrome (e.g. Dravet syndrome [DS]) and the underlying cause or epileptic disease (e.g. tuberous sclerosis complex, Rett and Angelman syndromes and neuronal migration abnormalities).
The ‘gold standard’ objective is to be able to ascribe a syndrome to every child with epilepsy. However, in practice, and even with the opportunity to observe a child’s epilepsy evolve, it is only possible to accurately identify a definite epilepsy syndrome in around 33–45% of children[13],
[14]
. It is important to avoid the temptation of trying to ascribe or ‘squeeze’ a child into a syndrome because if it is the wrong syndrome, it defeats the entire purpose and strength of the syndromic classification and gives the family potentially erroneous information about their child’s epilepsy.
Drug selection
The choice of AED is based primarily on:
- Known efficacy of the drug — in a specific epilepsy syndrome, or in the absence of an identified syndrome, the child’s seizure type(s);
- Known adverse side effects of the drug (idiosyncratic and dose-related);
- Dosing regime;
- Availability of child-friendly formulations;
- Known interactions with other AEDs (both synergistic and antagonistic) and other drugs;
- Some consideration of the cost of the drug and particularly the specific preparation.
There are few epilepsy syndromes, and even far fewer specific causes of the epilepsy, that respond to a clearly-identified AED or AED combination. The National Institute for Health and Care Excellence (NICE) has issued guidance on treatment approaches[15]
and examples include:
Syndrome: DS; the combination of sodium valproate, clobazam and stiripentol is generally considered to be the ‘best’ treatment regime for seizure control in children with this syndrome. Future research may identify a possible correlation between the specific mutation in the SCN1A gene that causes DS and response to one or more specific AEDs[15]
.
Disease: Infantile (epileptic) spasms (IS) caused by the neurocutaneous disorder tuberous sclerosis complex (TSC); vigabatrin completely suppresses the infantile spasms in at least 90% of children whose IS are caused by TSC and is the AED of first choice in this situation[15]
.
For most focal seizures and epilepsy syndromes that involve focal seizures (including those caused by a structural abnormality), carbamazepine or lamotrigine are the most effective AEDs[15]
; clinical experience indicates that vigabatrin may be particularly effective for focal epilepsies caused by a structural abnormality such as focal cortical dysplasia. For most generalised seizures, and epilepsy syndromes that involve generalised seizures (tonic-clonic, myoclonic and absence), sodium valproate (VPA) is the most effective AED[15]
.
In children aged under 12 years, dosing is based on body weight, although usually only up to a maximum of 50kg; adult dosing is generally used for children aged over 12 years and certainly over 14 years.
All AEDs have some pharmacokinetic and pharmacodynamic limitations, a spectrum of adverse side effects and teratogenicity, different formulations and costs. In addition, all may be subject to different expressions of genetically-determined pharmaco-resistance, a field of current active research. The vast majority of new AEDs will have received their initial licence for adjunctive or add-on use in adult patients with previously drug-resistant focal seizures. The definition of ‘adult’ has varied from 12 years (with gabapentin and, most recently, perampanel), 16 years (levetiracetam, lacosamide, eslicarbazepine) and 18 years (pregabalin, retigabine). Relatively few patients aged 12–16 years or 12–18 years were recruited into these adult studies because most patients within this age range will have been treated by paediatricians or paediatric neurologists and not by adult neurologists. Predictably, many AEDs used in children will be outside the drug’s product licence, which is common in paediatric prescribing. Finally, all anticonvulsants should be prescribed on a benefit-to-risk basis that will clearly vary between drugs and this will be determined by the evidence base; this may be influenced by clinicians’ experience and prejudice.
Prior to the publication of the first guideline on the diagnosis and management of epilepsies, published by NICE in March 2004[16]
, and the Scottish Intercollegiate Guideline Network (SIGN), published in 2005[17]
, the choice of AED in children was determined on a combination of evidence (largely anecdotal and non-randomised controlled trial [RCT] data), personal experience and prejudice. In January 2012, an updated NICE guideline incorporated new RCT data and introduced a health economic dimension to its treatment recommendations[15]
, a situation that, in 2015, led to a conflict with the advice issued by the Medicines and Healthcare products Regulatory Agency (MHRA) concerning VPA. Clear RCT evidence[18]
highlighted by NICE[15]
indicated that VPA is the most effective AED for the treatment of idiopathic (presumed genetic) generalised epilepsies (IGE) characterised by absence, myoclonic and tonic-clonic seizures. However, it may be associated with foetal teratogenicity[19]
. Consequently, the MHRA, following an edict from the Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency (EMA), wrote to all doctors in the UK stating: “Valproate medicines should not be used to treat epilepsy and bipolar disorder in girls, women who can become pregnant or pregnant women unless other treatments are ineffective or not tolerated.”[20]
This directive has caused considerable debate because the risks of foetal teratogenicity are primarily related to VPA taken with other AEDs, in a total daily dose exceeding 700mg. One view is that the doctor’s role is to provide accurate information about an AED, including any possible adverse side effects, following which the patient (and family) can make an informed choice, but adopting the MHRA’s directive would mean that all female patients (including children) with an IGE would be denied treatment with the most effective AED for their epilepsy.
‘Ideal’ antiepileptic drug
The ‘ideal’ antiepileptic drug (AED) has yet to be identified, but arguably should meet the following criteria:
- Effective against multiple seizure types (i.e. broad spectrum); this is important for syndromes characterised by multiple seizure types. VPA remains the clear ‘default’ AED of choice in the treatment of most of the IGE syndromes[15]
and Lennox-Gastaut and DS. Lamotrigine, levetiracetam and topiramate may be effective in IGE syndromes and topiramate and rufinamide in Lennox-Gastaut syndromes, but none have displaced VPA because of poorer efficacy or their association with more adverse side effects, or both. - No potentially life-threatening side effects.
- No or very few non-serious adverse effects; these may not be seen in early clinical trials and only emerge with longer-term, and a wider use, of new AEDs (e.g. felbamate [aplastic anaemia; hepatitis]; vigabatrin [constricted visual fields]; topiramate [cognitive-slowing; anorexia]; perampanel [mood and behaviour problems]).
- No idiosyncratic (allergic) reactions; anaphylaxis including Stevens-Johnson syndrome, which is potentially fatal (e.g. lamotrigine, carbamazepine).
- No or a limited dose-response relationship; this may appear a strange criterion for an ‘ideal’ AED, but it can be frustrating for both clinician and patient to prescribe and take a drug that has no known ceiling or maximum dose. The temptation is to continue increasing the dose in the hope or expectation that eventually seizure control will be achieved, assuming no adverse side effects have occurred precluding further dose increases.
- Acceptable drug preparations (e.g. a powder, dispersible tablet or appropriately flavoured liquid or intravenous preparation) that may be taken by all ages including infants/young children and those with learning difficulties (or other special needs). Specific situations include those where patients are unable to take oral medication (e.g. following surgery) and in individuals who present with acute seizures and require an immediate (intravenous) anticonvulsant that can then be continued intravenously or orally. Traditionally, these anticonvulsants have been phenobarbital, phenytoin or VPA, although phenobarbital and phenytoin are not popular or commonly-used long-term maintenance AEDs. Levetiracetam and VPA, which are available as intravenous preparations, may replace these drugs as the preferred second-line anticonvulsants because of their ‘cleaner’ safety profile and because they are more commonly used as long-term oral maintenance drugs.
- An acceptable dosing profile (once or no more than twice a day) as this will improve concordance.
- No significant interactions with other drugs (including other AEDs).
- No need to routinely measure the blood level of the AED (unless there is a concern over major non-concordance with medication or if the drug has complex pharmacokinetics/pharmacodynamics).
- No mutagenic or teratogenic effects (on the immature or developing organs and genetic tissue).
None of the current (old or new) AEDs meet all the criteria, and only a few meet some of these criteria.
Future pharmacotherapy
Traditional teaching has been that 25–30% of patients will never achieve at least a 12-month seizure-free period, despite the use of all currently-available AEDs[21]
. Although these data primarily relate to epilepsy in adults, they are broadly similar in children, although it will depend on the specific epilepsy syndrome. There remains the conviction that novel drugs must continue to be developed that are more effective or safer, or ideally both. Perhaps – but not necessarily. First, many reportedly drug-resistant patients will not have epilepsy; second, it is naïve to expect that all patients with epilepsy will achieve seizure freedom; this is reflected in the markedly heterogeneous nature and aetiology of the epilepsies; third, it is likely that some of the ‘old’ AEDs will have been used inappropriately for the child’s epilepsy syndrome (or seizure type), or for too short a period to adequately assess their effect.
New AEDs are likely to have been designed to answer a very narrow and short-term question within a trial protocol in a highly selective population, neither of which may be transferable to all patients with epilepsy. The longer-term use of the drug may demonstrate different efficacy and safety profiles, which may have a major effect on its survival in the AED market. The Standard And New Antiepileptic Drugs (SANAD) Study, published in 2007, provided a very useful template and, although not perfect, it illustrated how real-life and pragmatic studies can more appropriately address the needs of the larger population with epilepsy[18],
[22]
. Patients aged five years and over are currently being recruited to SANAD II, the results of which should be available in 2018.
Neurologists will continue to declare a need for new drugs to improve seizure freedom and the ‘quality of life’ of patients, and new ‘real life’ models of seizures and epileptogenesis will be identified. New drugs will act on the GABA system (gamma amino butyric acid), excitatory amino acids (e.g. the α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid [AMPA]) receptor of glutamate[23]
) or the various ion channels (on both the pre-synaptic and post-synaptic membrane) in all their types and configurations – with as yet unrecognised mechanisms of action. It is important to identify drugs that are neuroprotective and prevent chronic and medically-refractory epilepsy, such as epilepsies caused by brain trauma, infections of the central nervous system and following neonatal hypoxic-ischaemic encephalopathy. These novel AEDs will be prescribed in the hope that more patients will become seizure-free. Consequently, it will become even more difficult to decide when it is time to be empathetically honest with the patient and say that seizure freedom is not possible using conventional medical treatments. It is also likely that there will be a much closer cost-effective evaluation of new drugs, including AEDs, by groups such as NICE.
Newer AEDs have unquestionably improved the management of children with epilepsy. However, this wide choice of drugs may confuse inexperienced clinicians as to when and in what specific epilepsy syndrome, or even seizure type, these drugs should be prescribed, and in which order, and also which drugs to avoid. An additional potential consequence of having access to a larger choice of AEDs is that other potentially efficacious therapies, including the ketogenic diet and particularly surgery, may be inappropriately delayed while another drug to control a child’s refractory seizures is tried.
The newest and potentially most pharmacologically-challenging therapy in the treatment of the epilepsies is cannabis, or the medical cannabinoids, derived from Cannabis sativa (marijuana). A US parental-completed survey reported parents’ perceptions of the use of cannabidiol-enriched cannabis in 19 children aged 2–16 years with a paediatric drug-resistant epilepsy[24]
. Two patients became seizure-free, eight reported more than an 80% reduction in seizure frequency, and six reported a 25–60% reduction. The authors of this small, highly-subjective survey with limited follow-up concluded that “objective measurements of a standardised preparation of pure cannabidiol are needed to determine whether it is safe, well tolerated, and efficacious at controlling seizures in this paediatric population with difficult-to-treat seizures”. Another published anecdotal study lends support[25]
. In 2012, a Cochrane Review[26]
concluded that the evaluation of cannabinoids for treating epilepsy would demand “properly designed, high quality and adequately-powered trials”. Results from a number of international, open but also double-blind, placebo-controlled trials in DS and Lennox-Gastaut syndrome will be available in 2016.
Traditionally, the pharmaceutical industry has been reluctant to design and undertake paediatric RCTs. Clinicians have challenged this but only recently has this been taken up by the regulatory authorities, the US Food and Drug Administration (FDA) in the United States, the EMA and the MHRA. Drug studies in children are usually performed when there is considerable evidence of a new drug’s effectiveness, but perhaps more importantly its safety in adults. The National Institutes of Health in the United States, the EMA, and the ILAE have emphasised that much more should be known about the effects of all new AEDs at the approval stage regardless of the populations evaluated because of their use in children.
With few paediatric trials being conducted, it is estimated that approximately 40–50% of medicines prescribed to children in the EU are unauthorised for paediatric use[27],
[28]
. In the prospective European study, two thirds of children in hospital received at least one unlicensed or off-label drug, and almost half of all drug prescriptions were either unlicensed or off label[27]
. In 2006, the EU Regulation on Medicines for Paediatric Use indicated that the testing of new products in children should be mandatory if regulatory approval is to be granted for adults. This development process must include a Paediatric Investigation Plan (PIP), to be agreed with the EMA[29],
[30]
. It is recommended that a PIP should be submitted early during development so studies can be conducted in children, where appropriate, before marketing authorisation applications are submitted[31]
. This should mean that any new AEDs will be trialled in children and adults, and if not simultaneously, at least as soon as any adult efficacy and safety data are available. Finally, there is a need to study the efficacy and safety of any new AED in children with specific epilepsy syndromes, rather than simply seizure types.
Richard Appleton is paediatric neurology consultant at Alder Hey Children’s NHS Foundation Trust.
References
[1] Joint Epilepsy Council. Epilepsy prevalence, incidence and other statistics. September 2011. Available at: http://www.epilepsyscotland.org.uk/pdf/Joint_Epilepsy_Council_Prevalence_and_Incidence_September_11_(3).pdf (accessed November 2015).
[2] Office for National Statistics. Mid-2010 population estimates: United Kingdom; estimated resident population by single year of age. 2011. Available at: http://www.ons.gov.uk/ons/index.html (accessed November 2015).
[3] Camfield P & Camfield C. Incidence, prevalence and aetiology of seizures and epilepsy in children. Epileptic Disorders 2015;17:117–123. doi:10.1684/epd.2015.0736
[4] Meeraus WH, Petersen I, Chin RF et al. Childhood epilepsy recorded in primary care in the UK. Archives of Disease in Childhood 2013;98(3):195–202. doi:10.1136/archdischild-2012-302237
[5] Hindley D, Ali A & Robson C. Diagnoses made in a secondary care “fits, faints and funny turns” clinic. Archives of Disease in Childhood 2006;91(3):214–218. doi:10.1136/adc.2004.062455
[6] Uldall P, Alving J, Hansen LK et al. The misdiagnosis of epilepsy in children admitted to a tertiary epilepsy centre with paroxysmal events. Archives of Disease in Childhood 2006;91:219–221. doi:10.1136/adc.2004.064477
[7] Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised clinical and electrographic classification of epileptic seizures. Epilepsia 1981;22(4):489–501. doi:10.1111/j.1528-1157.1981.tb06159.x
[8] Commission on Classification and Terminology of the International League Against Epilepsy. Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia 1989;30(4):389–399. doi:10.1111/j.1528-1157.1989.tb05316.x
[9] Engel J. A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia 2001;42(6):796–803. doi:10.1046/j.1528-1157.2001.10401.x
[10] Engel J. Report of the ILAE Classification Core Group. Epilepsia 2006;47(9):1558–1568. doi:10.1111/j.1528-1167.2006.00215.x
[11] Berg AT, Berkovic SF, Brodie MJ et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia 2010;51(4):676–685. doi:10.1111/j.1528-1167.2010.02522.x (see also ‘invited commentaries’ on pages 713–724 of the same volume).
[12] Appleton RE. Classification in epilepsy: confusion, clarity or just new clothes for the emperor. Paediatric Epilepsy Current Awareness Service 2010;5(3):3–8. Available from: www.neuroeducation.org.uk
[13] Camfield P & Camfield C. Incidence, prevalence and aetiology of seizures and epilepsy in children. Epileptic Disorders 2015;17:117–123. doi:10.1684/epd.2015.0736
[14] Berg AT, Testa FM & Levy SR. Complete remission in nonsyndromic childhood-onset epilepsy. Annals of Neurology 2011;70(4):566–573. doi:10.1002/ana.22461
[15] National Institute for Health and Care Excellence. The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care. CG137. London: The National Institute for Health and Care Excellence, January 2012. Available at: http://www.nice.org.uk/guidance/cg137 (accessed November 2015).
[16] National Institute for Health and Care Excellence. The epilepsies: The diagnosis and management of the epilepsies in adults and children in primary and secondary care. CG20. London: The National Institute for Health and Care Excellence, October 2004. Available at: https://www.nice.org.uk/guidance/cg20
[17] Scottish Intercollegiate Guidelines Network. Guideline No. 81. Diagnosis and management of epilepsy in children and young people: a national clinical guideline. Edinburgh: Scottish Intercollegiate Guidelines Network, 2005. Available at: http://www.epilepsyscotland.org.uk/pdf/sign81.pdf (accessed November 2015).
[18] Marson AG, Al-Kharusi AM, Alwaidh M et al. The SANAD study of effectiveness of valproate, lamotrigine or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trial. Lancet 2007;369(9566):1016–1026. doi:10.1016/S0140-6736(07)60461-9
[19] Meador K, Reynolds MW, Crean S et al. Pregnancy outcomes in women with epilepsy: A systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Research 2008;81(1):1–13. doi:10.1016/j.eplepsyres.2008.04.022
[20] Medicines and Healthcare products Regulatory Agency. Drug safety update. Medicines related to valproate: risk of abnormal pregnancy outcomes. 2015. Available at: https://www.gov.uk/drug-safety-update/medicines-related-to-valproate-risk-of-abnormal-pregnancy-outcomes (accessed November 2015).
[21] Kwan P, Schachter SC & Brodie MJ. Drug-resistant epilepsy. New England Journal of Medicine 2011;365(10):919–926. doi:10.1056/nejmra1004418
[22] Marson AG, Al-Kharusi AM, Alwaidh M et al. The SANAD Study of efficacy of carbamazepine, gabapentin, lamotrigine, oxcarbazepine or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet 2007;369(9965):1000–1015. doi:10.1016/s0140-6736(07)60460-7
[23] Steinhoff BJ. The AMPA receptor antagonist perampanel in the adjunctive treatment of partial-onset seizures: clinical trial evidence and experience. Therapeutic Advances in Neurological Disorders 2015:8(3):137–147. doi:10.1177/1756285615575696
[24] Porter BE & Jacobson C. Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy. Epilepsy and Behaviour 2013;29(3):574–577. doi:10.1016/j.yebeh.2013.08.037
[25] Press CA, Knupp KG & Chapman KE. Parental reporting of response to oral cannabis extracts for treatment of refractory epilepsy. Epilepsy and Behaviour 2015;45(49):49–52. doi:10.1016/j.yebeh.2015.02.043
[26] Gloss D & Vickrey B. Cannabinoids for epilepsy. Cochrane Database of Systematic Reviews 2012. doi:10.1002/14651858.CD009270.pub2
[27] Conroy S. Survey of unlicensed and off-label drug use in paediatric wards in European countries. BMJ 2000;320(7227):79–82. doi:10.1136/bmj.320.7227.79
[28] Palmaro A, Bissuel R, Renaud N et al. Off-label prescribing in pediatric outpatients. Pediatrics 2015;135(1):49–58. doi:10.1542/peds.2014-0764
[29] Medicines and Healthcare products Regulatory Agency. Legal requirements for children’s medicines. 2014. Available at: https://www.gov.uk/government/publications/legal-requirements-for-childrens-medicines (accessed November 2015).
[30] European Commission. Medicines for children — PIP guideline. Available at: http://ec.europa.eu/health/human-use/paediatric-medicines/developments/consultation-responses_en.htm (accessed November 2015).
[31] Appleton RE & Marson AG. Anti-epileptic drug trials in children: plus ca change? Paediatric Epilepsy Current Awareness Service 2012;7(4):12–18. Available at: www.neuroeducation.org.uk.