Maurizio de Angeles / Science Photo Library
Targeting the immune system as a means of activating anti-tumour activity through immune checkpoint inhibition is proving to be an increasingly effective method of treating a variety of cancers, prolonging life and increasing progression-free survival. Consequently, immune checkpoint inhibitors are available for a number of different indications, with the potential of being licensed for further indications in the future (see Table 1).
Increased survival benefits have been demonstrated in a number of different studies. A study by Wolchok et al., which aimed to determine the overall survival benefit of combined treatment with nivolumab and ipilimumab in patients with advanced melanoma, demonstrated a 58% survival at three years in the combination arm of the trial[1]
. The recent phase III study by Motzer et al. demonstrated that patients with advanced renal cell carcinoma given a combination of nivolumab and ipilimumab had an increased survival versus sunitinib (a multi-targeted receptor tyrosine kinase inhibitor) at 18 months (75% overall survival versus 60%, respectively)[2]
. These results have helped pave the way for the development of new National Institute for Health and Care Excellence (NICE) guidance for this indication and also demonstrate the rapidly changing indications available for these medicines (see Table 1).
Owing to their mechanism of action, immune checkpoint inhibitors are associated with autoimmune-related side effects and toxicities. Therefore, it is vital that pharmacists and healthcare professionals are able to effectively recognise and manage these toxicities, not only because of their potential severity, but also because their prevalence can be very high. For example, 95% of patients experienced some form of treatment-related adverse event when receiving combination nivolumab and ipilimumab for metastatic melanoma[3]
.
This article describes the immune checkpoint inhibitors currently available, and how pharmacists and healthcare professionals can recognise and manage the common toxicities associated with their use.
T-cell activation
Immunotherapy differs from more traditional methods of cancer cell killing via cytotoxic/chemotherapy drugs (e.g. cisplatin, methotrexate) or by targeting growth signalling pathways in cancer cells (e.g. osimertinib, ceritinib), by exploiting a patient’s immune system to destroy tumour cells[4],[5]
.
T-cell activation (both the amplitude and quality of immune response) is initiated by T-cell receptors that recognise antigens: a process controlled by a balance between inhibitory (immune checkpoints) and stimulatory signals. Consequently, the immune system can maintain self-control or self-tolerance (i.e. preventing autoimmune responses) while protecting tissue from damage when responding to an antigen-expressing pathogen or tumour cell[5],[6]
.
However, cancer cells exploit immune checkpoints by ‘switching off’ the immune system, allowing cancer cells to avoid immune system attack. Tumour cells ‘hijack’ certain immune checkpoint pathways that would normally lead to activation of T cells specific for tumour antigens and tumour cell destruction[6]
.
Pharmacology of immune checkpoint inhibitors
Immune checkpoint inhibitors are negative regulatory molecules often found on the surface of T cells. They regulate responses from self-proteins and protect against autoimmune activity[6]
. They prevent the ‘switching off’ of the immune system and, thereby, allow T cells to attack cancer cells (see Figure 1). As a result, there is increased activation of the immune system; however, because this action is not selective, the inhibition of these self-regulatory checkpoints can lead to autoimmune-related toxicities[7],[8],[9]
. These adverse events can be categorised as infusion-related toxicities and immune-related adverse events (irAEs), where the latter ranges from relatively mild to potentially life-threatening effects[10]
. The frequency and type of toxicities that can occur depend on the checkpoint being targeted.
The inhibitory immune checkpoint pathways that have drugs approved for use in cancer are cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed death receptor-1 (PD-1), as well as the ligand of this receptor (PD-L1).
NICE-approved indications for immune checkpoint inhibitors at the time of writing are listed in Table 1[11]
; however, it is important to note that new indications may continue to become available. This list also contains indications that, at time of writing, have been proposed and may be approved in the coming months (listed as “unlicensed”), and those in development that have been approved but full guidance has yet to be published (also listed as “unlicensed”).
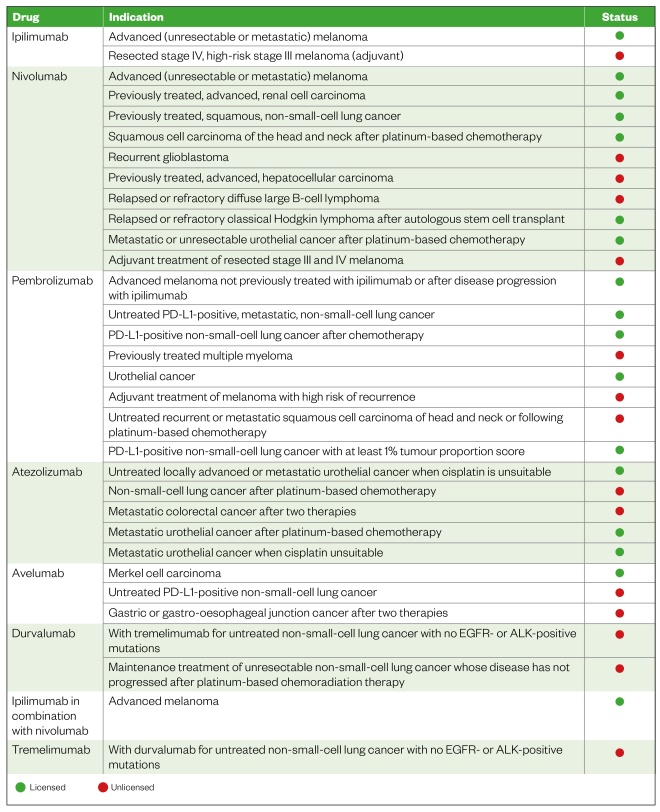
Table 1: Immune checkpoint inhibitors and their proposed and NICE-approved indications
There are many other immune checkpoints that could become future targets[4]
, but further research and drug discovery in this area are required.
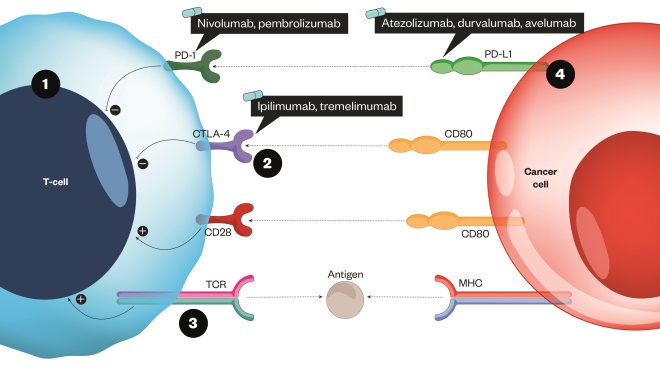
Figure 1: How immune checkpoint inhibitors can help facilitate increased cancer cell death
Source: MAG / The Pharmaceutical Journal
The T-cell receptor binds to an antigen found on the major histocompatibility complex on the surface of the cancer cell.
1) This is a stimulatory response and activates T cells to remove pathogens or cancer cells (shown as the positive circles). A co-stimulatory receptor also exists (CD28), which binds to a ligand (CD80). This results in an increased immune response toward the cancer cell
2) CTLA-4 has a stronger affinity to CD80 and so competes with the co-stimulatory pathway to inhibit the response and ‘switch it off’
3) When a strong TCR stimulus exists, the inhibitor molecule CTLA-4 is upregulated and transported to the surface of the cell; a similar process occurs with PD-1
4) The checkpoint inhibitors act by blocking the inhibitory response by targeting CTLA-4, PD-1 or the ligand PD-L1[8]
Toxicities
The nature and severity of immune-related toxicities can vary greatly depending on the patient, the drugs or combination of drugs that are used. For infusion-related reactions, guidance exists in the drug’s summary of product characteristics, but this generally results in the withdrawal of the drug in moderate to severe cases of hypersensitivity. If milder reactions are experienced, future cycles of therapy may continue with close patient monitoring accompanied by appropriate premedication, according to local guidance for prophylaxis of infusion-related reactions. This typically involves treatment with antipyretic and antihistamine medication.
Immune-related toxicities are graded using the National Cancer Institute’s common toxicity criteria[12]
. This is done using a grading number system from one to four — grade one being a relatively mild reaction, and grade four being very serious or life threatening. When considering individual drugs, studies have shown that the agents used can have an effect of severity, incidence and type of reaction[10],[13]
.
For instance, CTLA-4 inhibition irAEs (e.g. with ipilimumab) have been recorded in 60–85% of patients[9],[14]
. Around 10–27% of patients developed grade three or four reactions[10]
. The most common reactions included rashes (which were typically the first to develop), liver toxicity, diarrhoea and hypophysitis. Peak onset of adverse reactions of ipilimumab was around eight weeks from initiation[10]
.
PD-1/PD-L1 inhibition differs from this, in that the most common irAE is fatigue. Incidence differs according to individual studies but is around 12–37% depending on dose and whether the drug is PD-1 or the ligand PD-L1[10]
. For nivolumab, adverse events have been documented in 74–85% of patients. A range of grade three and four toxicities were experienced (depending on the diagnosis of the patients being treated), but could be up to 79% and 19%, respectively. Pembrolizumab was shown to cause irAEs in up to 80% of patients. Grade three or four toxicities have been documented in 13–16% of patients, but are again dependent on dose and the indication being treated[10],[14]
. Combination treatments currently only licensed in melanoma can illicit higher rates of irAEs[3],[10]
. It should be noted that, overall, irAEs for checkpoint inhibitors are dose dependent and the incidence varies according to indication.
Managing toxicities
Guidance from the European Society of Medical Oncology, published literature and local guidelines[10],[13],[15],[16]
should all be considered when managing toxicities, as each contains in-depth treatment algorithms for each irAE depending on the grade of toxicity experienced. In general, toxicities are dermatological, endocrine, hepatic, gastrointestinal or respiratory in nature (see Figure 2).
There are commonalities with regard to the treatment of toxicities; mild reactions typically involve symptomatic relief, whereas moderate reactions require corticosteroid treatment (or infliximab for more severe reactions; see Figure 2).
The grade of reaction dictates whether the therapy should be withdrawn or withheld for a period of time. It should also be noted that irAEs can present at varying stages of treatment, including after treatment cessation[10],[13],[15],[16]
.
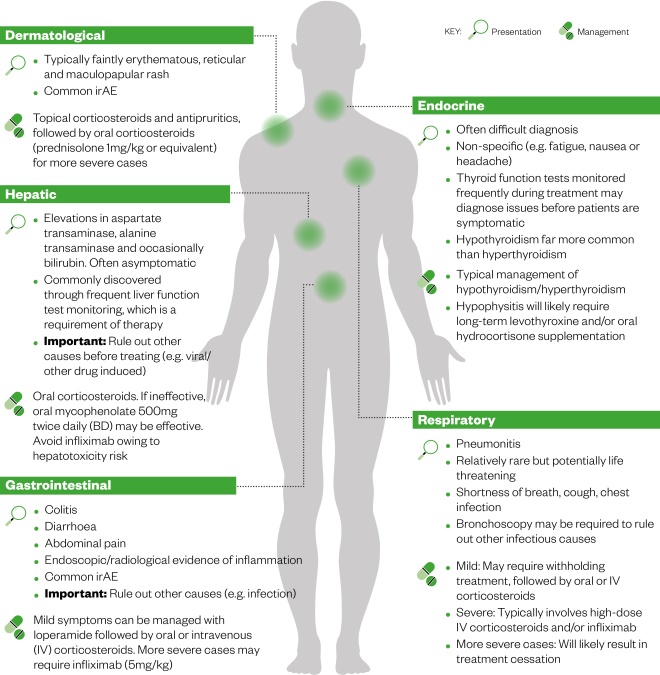
Figure 2: The most common immune-related adverse events (irAEs), their presentation and management[10],[13],[15],[16]
Source: MAG / The Pharmaceutical Journal
In general, toxicities are dermatological, endocrine, hepatic, gastrointestinal or respiratory in nature. There are also commonalities with regard to the treatment of toxicities; mild reactions typically involve symptomatic relief, whereas moderate reactions require corticosteroid treatment (or infliximab for more severe reactions).
The role of the pharmacy team
As immune checkpoint inhibitors become licensed for more indications, the pharmacy team may more frequently encounter patients in a variety of clinical settings who are receiving these treatments. Therefore, it is important to understand immune checkpoint inhibitors in terms of incidence of irAEs, treatment and relevant signposting.
If a person with cancer is presenting with any of the symptoms featured in Figure 2, they should be questioned about the type of therapy they are receiving or have previously received. All patients should be advised to immediately report any of these signs and symptoms to their local unit.
Information sources are available to inform frontline healthcare professionals, as well as patients, about the adverse event profile of immune checkpoint inhibitors and how they differ in both outcome and treatment when compared with conventional chemotherapy (see useful resources box). All healthcare professionals should have a low suspicion that any patient presenting may have a toxicity to an immune checkpoint inhibitor. Any confirmed or suspected irAEs should be reported via the Medicines and Healthcare Products Regulatory Agency’s Yellow Card reporting scheme[17]
.
Pharmacists and healthcare professionals should also signpost patients to appropriate help. Cancer units provide in-house contact details that are accessible seven days a week, and all immune checkpoint inhibitor patients should be encouraged to report all adverse events, no matter how mild. Many cancer centres have clinical nurse specialists or cancer key workers who can offer advice.
Useful resources
- Cancer Research UK
- Macmillan drug information sheets
- Understanding immunotherapy side effects— an infographic from the Comprehensive Cancer Network and the American Society of Clinical Oncologists
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] Wolchok JD, Chiarion-Sileni V, Gonzalez R et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684
[2] Motzer RJ, Tannir NM, McDermott DF et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126
[3] Larkin J, Chiarion Sileni V, Gonzalez R et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:1270–1271. doi: 10.1056/NEJMc1509660
[4] Sheng J, Srivastava S, Sanghavi K et al. Clinical pharmacology considerations for the development of immune checkpoint inhibitors. J Clin Pharmacol 2017;57(Suppl 10):S26–S42. doi: 10.1002/jcph.990
[5] Lee L, Gupta M & Sahasranaman S. Immune checkpoint inhibitors: an introduction to the next-generation cancer immunotherapy. J Clin Pharmacol 2016;56(2):157–169. doi: 10.1002/jcph.591
[6] Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12(4):252–264. doi: 10.1038/nrc3239
[7] Passardi A, Canale M, Valgiusti M & Ulivi P. Immune checkpoints as a target for colorectal cancer treatment. Int J Mol Sci 2017;18(6):1324. doi: 10.3390/ijms18061324
[8] Buchbinder EI & Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol 2016;39(1):98–106. doi: 10.1097/COC.0000000000000239
[9] Hodi FS, O’Day SJ, McDermott DF et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–723. doi: 10.1056/NEJMoa1003466
[10] Haanen J, Carbonnel F, Robert C et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines. Ann Oncol 2017;28(Suppl 4):iv119–iv142. doi: 10.1093/annonc/mdx225
[11] National Institute for Health and Care Excellence. Guidance and advice list. Available at: https://www.nice.org.uk/guidance/published?type=apg,csg,cg,mpg,ph,sg,sc (accessed May 2018)
[12] National Cancer Institute. Common toxicity criteria manual. Available at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcmanual_v4_10-4-99.pdf#search=%22common%20toxicity%20criteria%22 (accessed May 2018)
[13] Friedman C & Postow M. Managing immunotherapy-related side effects. Oncol Hematol Rev 2015;11(2):143–144. doi: 10.17925/OHR.2015.11.02.143
[14] Herbst RS, Baas P, Kim DW et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7
[15] Naidoo J, Page DB, Li BT et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 2015;26(12):2375–2391. doi: 10.1093/annonc/mdv383
[16] Horvat TZ, Adel NG, Dang T et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 2015;33(28):3193–3198. doi: 10.1200/JCO.2015.60.8448
[17] Medicines and Healthcare Products Regulatory Agency (MHRA). The Yellow Card Scheme: guidance for healthcare professionals, 2017. Available at: https://www.gov.uk/guidance/the-yellow-card-scheme-guidance-for-healthcare-professionals (accessed May 2018)


