Shutterstock.com
In this article you will learn:
- The different approaches taken to determine the correct dose of gentamicin
- How to calculate ideal body weight and corrected body weight
- How to monitor patients receiving gentamicin to reduce risk of side effects
Gentamicin is an aminoglycoside antibiotic commonly used for the treatment of infections and surgical prophylaxis. It is not absorbed from the gut when administered orally, and is therefore predominantly administered via intramuscular or intravenous injection. Gentamicin can cause serious dose-related side effects including nephrotoxicity and irreversible hearing loss, so it is important to ensure patients receive the correct dose and are monitored regularly.
Indications
Aminoglycosides have bactericidal activity for some Gram-positive and most aerobic and facultative anaerobic Gram-negative bacteria. They act by inhibiting protein synthesis. Once inside the bacterial cell, they bind to the 30S subunit of the ribosome causing misreading of mRNA, resulting in interruption of normal bacterial protein synthesis[1]
. As aminoglycoside uptake into bacteria is oxygen dependent, they are therefore not active against anaerobes.
Gentamicin is commonly used to treat urinary tract infections, sepsis, intra-abdominal infections, endocarditis, pelvic inflammatory disease and complicated skin, bone and soft tissue infections. It is often used for more serious Gram-negative infections, or in combination with a broad spectrum beta lactam antibiotic to provide coverage against Gram-positive bacteria.
Gram-negative bacteria can be difficult to treat because of the complex nature of their cell wall. Beta lactam antibiotics active against the cell wall (e.g. piperacillin/tazobactam) can be taken simultaneously to facilitate aminoglycoside penetration into the cell, increasing efficacy and resilience[2],
[3]
.
Amino
glycosides may cause auditory or vestibular nerve damage in infants if used during the second or third trimesters of pregnancy, and should be avoided if possible. If treatment is required, gentamicin is the preferred aminoglycoside because, although it does cross the placenta, it has not been associated with developmental toxicity[4]
. It can also be used in patients who are breastfeeding[5]
.
Gentamicin is contraindicated in patients with myasthenia gravis, where neuromuscular transmission may be impaired. It should also be avoided in patients taking drugs that can cause nephrotoxicity (e.g. ciclosporin, amphotericin B) and ototoxicity (e.g. furosemide).
Dose
Gentamicin is usually given by slow bolus injection over two to three minutes or via intravenous infusion over 30 minutes.
A variety of dosing regimens ar
e available, all of which use a corrected version of the patient’s weight when calculating doses. Ideal body weight (IBW) should be used in all non-obese patients, unless actual body weight is lower; in these patients actual body weight should be used.
Aminoglycosides are not distributed into adipose tissue, as they are highly hydrophilic. Therefore corrected body weight (CBW) should be used for dosing calculations for obese patients, rather than IBW (see ‘Gentamicin body weight calculations’).
Gentamicin and other aminoglycosides are cleared by the kidneys, and it is therefore important that renal function is assessed before treatment starts. It is important to ensure an accurate renal function value is used to reduce the risk of toxicity. For overweight patients, IBWshould be used when calculating renal function.
Gentamicin body weight calculations
The majority of patients given gentamicin will use the following calcuations, rather than their actual body weight (ABW).
Ideal body weight (IBW)
IBW Male (kg) = 50 + (2.3 x number of inches above five feet in height)
IBW Female (kg) = 45.4 + (2.3 x number of inches above five feet in height)
Corrected body weight (CBW)
CBW = IBW + 0.4 (ABW – IBW)
For example, an obese male patient who is 6’1” tall and weighs 120kg would result in the following calculation:
IBW = 50 + (2.3 x 13) = 79.9kg
CBW = 79.9 + 0.4 (120 – 79.9) = 95.9kg when calculating dose.
Gentamicin’s bactericidal activity is concentration-dependent, and treatment should aim for a peak concentration of eight to ten times the mean inhibitory concentratio
n (MIC).
Aminoglycosides also have a significant post-antibiotic effect and cause inhibition of bacterial growth after only a brief exposure. This
means periods of low drug concentrations are used to minimise drug toxicity without reducing efficacy.
Extended interval regimens (e.g. once daily dosing) can also be used over more traditional multiple daily dosing for the treatment of the majority of infections, reducing the potential for toxicity. This has largely superseded multiple daily dose regimens, and includes 5mg/kg dosing, using the Hartford nomogram, and individualised approaches.
Extended interval regimens may not be suitable in pregnant patients or those with endocarditis because there is a lack of evidence of using this dosing regimen in these patients. Extended interval regimens should also be avoided in patients with burns of more than 20% of the total body surface area, or patients with a creatinine clearance of less than 20ml/min.
Other aminoglycosides (e.g. amikacin, streptomycin and tobramycin) can be dosed using similar methods, but use different dose-weight ratios.
| Table 1: Patient groups potentially requiring increased doses | |
|---|---|
Patient group | Rationale for increased doses |
Cystic fibrosis | Increased extracellular fluid, leading to increased volume of distribution, increased rates of elimination (up to 50%) |
Major burns | Increased rates of elimination |
Intensive care | Increased volume of distribution and hypermetabolic state |
Ascites | Increased extracellular fluid, leading to increased volume of distribution |
Obese | Increased extracellular fluid, leading to increased volume of distribution. These patients should be closely monitored, as it is easy to underdose and overdose |
Multiple daily dose regimens
involve a total d
aily dose of gentamicin of 3–5mg/kg, divided into three doses. These doses should be given by intramuscular or slow intravenous injection over at least three minutes; infusions should not be used as this could make the peak level less accurate. The most commonly used dose is 80mg three times daily. Pre-dose (trough) gentamicin levels should be checked after 24 hours of treatment (target <2mg/L) and peak levels measured one hour post-dose (target 5–10mg/L). Some infections, such as
Pseudomonas spp.may require an increased dose to achieve higher peak levels.
5mg/kg dosing
involves giving a p
atient an initial dose of intravenous gentamicin of 5mg/kg, unless creatinine clearance is <20ml/min, when a reduced dose (e.g. 2–3mg/kg) should be used. Baseline creatinine clearance should be checked for all patients (see ‘Monitoring requirements’). Using lower doses of gentamicin in patients with renal impairment to reduce toxicity has no effect on the efficacy of treatment[6]
.
The frequency of dosing and timing of the next dose depends on the patient’s renal function. If the patient’s creatinine clearance is >60ml/min, doses should be given every 24 hours. This is extended to every 36 hours in those with a creatinine clearance of 40–59ml/min; and every 48 hours in those with a clearance of 20–39ml/min. Patients with a creatinine clearance of <20ml/min should have gentamicin levels taken every 48 hours, and receive their next dose when levels fall to <1mg/L.
Pre-dose (trough) gentamicin levels should be taken four hours before the next dose is due. Pre-dose levels of <1mg/L are required before the next dose can be given, in order to minimise toxicity. If the trough level is above 1mg/L the dose must be omitted until the level falls below 1mg/L. Measurement of peak levels are not usually necessary during 5
mg/kg dosing.
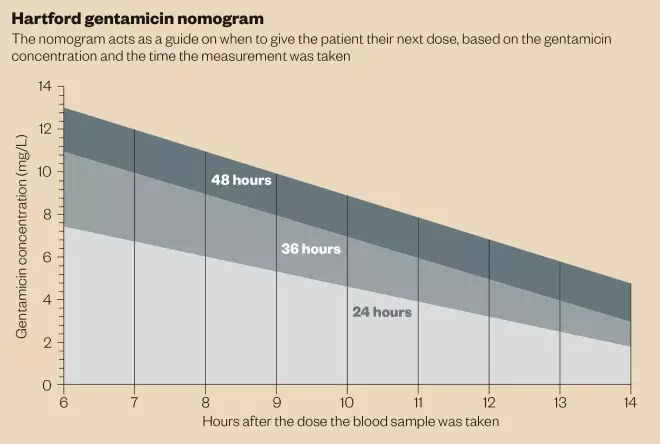
Hartford
nomogram regimens are based on the results of an American study where patients received 7mg/kg of gentamicin, and received the next dose based on estimated creatinine clearance. Reportedly only 27 patients (1.2%) developed nephrotoxicity and three patients developed ototoxicity[7]
. It should not be used in patients with a creatinine clearance of <20ml/min.
The first dose of gentamicin should be administered at 7mg/kg, and gentamicin levels should be taken 6–14 hours after the first dose. This level should be compared with the Hartford nomogram (see ‘Hartford gentamicin nomogram’) to determine the appropriate dosing interval to use. For example, if, after nine hours, a patient’s serum gentamicin level was 4mg/L, they should receive a dose every 24 hours; if the serum concentration was 8mg/L, the dose should be given every 36 hours.
If the concentration of gentamicin falls above the 48 hour line, the Hartford nomogram may not be an appropriate dosing regimen to use and individualised pharmacokinetic dosing should be considered. If gentamicin is to be continued regardless, the next dose should not be administered until the gentamicin blood level is <
2mg/L.
If renal function remains constant, gentamicin levels should be monitored twice weekly and doses adjusted accordingly.
Synergy regimens(e.g., with beta lactam antibiotics for endocarditis), involve 1mg/kg of intravenous gentamicin being administered every eight hours. Pre-dose (trough) gentamicin levels should be checked after 24 hours and then twice weekly (target <1mg/L). Peak gentamicin levels, taken one hour after administration, can also be measured (target 3–5mg/L).
Individualised dosing based on the patient’s pharmacokinetics can also be used. This means treatment is tailored to the patient, ensuring accurate dosing, improved efficacy, and reduced potential for toxicity. It is also useful for patients who are renally impaired. However, this approach is complex, requiring extensive input from a team of clinical pharmacists as part of a 24-hour service, and requires accurate recording of the time doses are administered and levels are taken regularly, to ensure data are interpreted correctly.
| Table 2: Monitoring requirements | ||
|---|---|---|
Monitoring required | Rationale | Frequency of monitoring |
Drug levels | Efficacy and minimising side effect risk. | Initially daily, but can move to twice weekly if the patient is stable on treatment. |
Renal function | Clearance of aminoglycosides is dependent on renal function. | If baseline renal function is normal, monitor the patient twice weekly. If baseline renal function is deranged, or renal function deteriorates, monitor the patient daily whilst on treatment. |
Weight | Fluctuations in weight may affect dose. | Baseline and then weekly weights. |
Auditory function | Ototoxicity and vestibulotoxicity can occur and are irreversible side effects. | Baseline and weekly testing. |
Side effects and monitoring
All patients s
hould have a kidney function test before starting gentamicin, and renal function should be assessed regularly (see ‘Monitoring requirements’). Premature infants and neonates need extensive monitoring if prescribed gentamicin because of their renal immaturity. If renal function is reduced during treatment, the dose of aminoglycoside should be adjusted accordingly.
Fluid balance should be closely monitored and dehydration must be corrected prior to commencing treatment. Blood serum levels should be measured frequently, especially in patients with renal impairment and the elderly. These should not be taken from the line being used for drug administration.
The main side effects of gentamicin are dose related. Harmful side effects are ototoxicity, which is irreversible, and nephroptoxicity. Patients should be counselled to report adverse effects, such as dizziness, nausea and hearing loss, without delay.
Side effects and toxicity are closely related to prolonged duration of treatment. Where possible, treatment with aminoglycosides should be limited and not exceed seven days. The “start smart then focus” guidelines from Public Health England recommend that antibiotics are reviewed at 48–72 hours after starting treatment, and then if treatment is to be continued, a further review or date to stop treatment should be documented[8]
. The Brit
ish National Formulary recommends that the duration of treatment should not exceed seven days.
Fran Garraghan is acting lead antimicrobial pharmacist and Rachael Fallon is deputy director of pharmacy at Central Manchester University Hospitals NHS Trust.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] Mingeot-Leclercq M, Glupczynski Y & Tulkens P. Aminoglycosides: activity and resistance. Antimicrobial Agents and Chemotherapy 1999;43(40):727–737.
[2] Doi Y & Arakawa Y. 16S Ribosomal RNA Methylation: emerging resistance mechanism against aminoglycosides. Clinical Infectious Diseases. 2008;45(1):88–94.
[3] Hollenbeck B & Rice L. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence 2012;3(5):421–569.
[4] Briggs G & Freeman RK. Lippincott, Williams & Wilkins. Drugs in Pregnancy and Lactation 10th Edition.
[5] US National Library of Medicine. Lactmed. Available at: http://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm (accessed 25 July 2015).
[6] Feliu C, Wynckel A, Gaha K et al. Evaluation of efficacy on the basis of the ratio Cmax/IMC and toxicity of low dose of gentamicin in patients with renal impairment. Fundamental and Clinical Pharmacology 2015;29(10):0767–3981.
[7] Nicolau D, Freeman C, Beliveau P et al. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrobial Agents and Chemotherapy 1995;39(3):650–655.
[8] Public Health England. Start smart then focus, antimicrobial stewardship toolkit for English hospitals. Updated March 2015. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/417032/Start_Smart_Then_Focus_FINAL.PDF (accessed 25 July 2015).
You might also be interested in…

EMA may ask manufacturers to increase production capacity in response to medicines shortages

Meeting May 2024 Pharmacy First payment thresholds is ‘an area of risk’, warns Community Pharmacy England

