This content was published in 2012. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
For all newborns admitted to a neonatal intensive care unit (premature babies and sick full-term infants), care can be divided into two general components: supportive (i.e., care required to maintain as near to normal homeostatic function as possible) and reactive (i.e., treatment started in response to illness or change in an infant’s health). Broadly, supportive care encompasses the following:
- Respiratory support;
- Cardiovascular support;
- Nutritional support.
Reactive care generally involves treatment ofcomplications, namely:
- Neurological issues (e.g., seizures);
- Patent ductus arteriosus;
- Necrotising enterocolitis;
- Retinopathy of prematurity.
Respiratory support
The fetal lung is not structurally mature until around 35 weeks’ gestation[1]. Respiratory failure can be caused by incomplete lung development or surfactant deficiency, and is a major cause of morbidity and mortality in preterm infants[2]. The initial support given depends on the gestational age at birth and clinical condition of the infant. The earlier the gestation at birth, the higher the likelihood that prolonged respiratory support will be required. There are various aspects of mechanical and pharmacological respiratory support.
Ventilation
Ventilation is used to support infants who are unable to breathe effectively for themselves. It can be invasive (the baby is attached to a ventilator via an endotracheal tube) or non-invasive (the baby is connected to a pressure support system via nasal prongs or a soft facemask). Although ventilation is the mainstay of non-pharmacological human respiratory support, its efficacy can be enhanced by the use of certain medicines, outlined below.
Surfactant replacement therapy
Pulmonary surfactant is produced in the lungs, but is often deficient in premature babies. Early administration of surfactant (via an endotracheal tube) for infants who are likely to be surfactant deficient reduces their initial oxygen and ventilation requirements. It also reduces the incidence of respiratory distress syndrome (RDS), pneumothorax, pulmonary interstitial emphysema and death[2]. Surfactant therapy is also used in full-term neonates who have meconium aspiration syndrome. The surfactants used in the UK are poractant alfa and beractant, which are modified and purified from porcine lung and bovine lung, respectively. Natural surfactants have a more rapid onset of action, and are associated with improved survival, compared with the synthetic products that were used in the past[3]. Antenatal corticosteroid use reduces the incidence of neonatal RDS by promoting surfactant production. A combination of antenatal corticosteroid administration and postnatal surfactant replacement therapy has been demonstrated to improve neonatal lung function more than either treatment alone[2].
Postnatal corticosteroids
The use of corticosteroids in the neonatal period can facilitate weaning from intensive ventilatory support. However, they are used sparingly due to adverse effects on cognitive and motor development. Children who are treated with dexamethasone after premature birth have been shown to perform significantly worse in cognitive and motor testing at school age compared with preterm infants who are not treated with corticosteroids[4]. Corticosteroid use is also associated with an increase in risk of secondary infection[3].
According to a 2008 review, hydrocortisone appears to be an effective alternative to dexamethasone, with fewer long-term adverse effects (neurological, neuro-endocrine and cardiovascular). However, trial numbers are small and blinded randomised controlled trials are needed to confirm these findings[5].
Caffeine
Neonatal apnoea due to immaturity of the cerebral respiratory centre can delay progression from invasive to non-invasive respiratory support. Caffeine —a respiratory stimulant — can be used for babies with neonatal apnoea to facilitate respiratory weaning earlier than might otherwise be achieved, and may even allow a premature baby to avoid the need for invasive support at all. A loading dose achieves therapeutic serum caffeine levels quickly; smaller subsequent doses, given once or twice daily, maintain serum levels until the respiratory centre is fully mature (at 33–34 weeks’ corrected gestation, see Box 1)[3]. The “Caffeine therapy for apnoeas of prematurity” study showed that infants who received caffeine were weaned from invasive ventilation a week earlier than those who received placebo. Moreover, fewer babies who received caffeine required supplemental oxygen at 36 weeks’ corrected age[6]. There is 1mg of caffeine base in 2mg of caffeine citrate so, to avoid errors, it is important that clinicians indicate whether a dose is prescribed in terms of caffeine base or caffeine citrate[3]. The British National Formulary for Children recommends that the dose is prescribed in terms of caffeine base.
Box 1. Terminology regarding preterm age
Gestational age is the time elapsed between the first day of the last normal menstrual period and the day of delivery. It is defined in weeks and days, for example 25 weeks and 2 days would be expressed as 25+2. Corrected gestational age is calculated as the gestational age plus the chronological age of the infant. For example, a baby born at 25 weeks and 2 days, who is now 1 week and 4 days old, would have a corrected gestational age of 26 weeks and 6 days, expressed as 26+6.
Cardiovascular support
Cardiovascular support in sick neonates is usually started on the basis of clinical and echocardiographic assessment. Examples of such support include the use of vasopressors and fluid administration for neonates with sepsis and the use of cardioselective inotropes following perinatal asphyxial myocardial damage. Historically, a combination of dopamine and dobutamine has been used first line, with adrenaline added if required. A one-off dose of hydrocortisone can begiven if inotrope resistance occurs. Other inotropes can also be used in specific clinical circumstances; choice of drug will depend on action and receptor profile[7]. Vasoconstrictive drugs may have undesirable side effects in flow-sensitive, maturing organs (e.g., brain, kidney) and therefore the risks and benefits of their use must be weighed up.
There is a lack of consensus about how measurement of cardiovascular status can be reproduced reliably to predict long-term outcome.
Nutritional support
Poor nutritional status can have a negative impact on the survival of preterm infants, and is linked with an increased risk of necrotising enterocolitis, septicaemia, impaired growth and poor cognitive outcome[8].
Stores of every major nutrient are built up by a foetus in the later stages of a normal pregnancy; therefore, infants born preterm have not undergone this process. A preterm baby weighing 1kg has sufficient energy stored to survive for two to three days; death occurs if nutritional replacement is not supplied during and after this period[8].
The metabolic demands of preterm infants are high from the moment of delivery. However, it is not possible to start most preterm infants on enteral feeds alone because the immature gut will not tolerate the quantities that would be required to provide complete nutrition. Total parenteral nutrition is often used as a nutritional bridge between birth and the time from which enteral feeds alone can provide full nutrition, or for those who are nil-by-mouth.
Breast milk is the most complete form of nutrition for infants over 1.5kg. It reduces the risk of necrotising enterocolitis and infections, and improves cognitive outcome[8]. Colostrum is the first milk produced by the mother and it contains antibodies which provide passive immunity for the neonate and first protection against pathogens. Mature breast milk contains high levels of prebiotics and probiotics (promoting the growth of “good” gut bacteria, which help immune function)[8] as well as optimal amounts of fat, carbohydrate and salt.
Although expressed breast milk is the best milk to give enterally to any preterm infant, it will not fully meet the nutritional demands of very preterm infants even if given in large volumes. In these situations, breast milk fortifier can be added to boost the protein and calorie content of the breast milk[8]. In situations where expressed breast milk is not available, preterm-specific formulas should be used; standard baby formulas do not meet the needs of preterm infants.
Vitamin and mineral supplementation
Although micronutrient and mineral supplementation varies between neonatal units across the UK, there is some consistency for a few key supplements. Phosphate is essential for bone growth and mineralisation, and deficiency occurs frequently in preterm infants. Preterm infants fed exclusively on breastmilk, which is relatively phosphate-poor, should receive an oral phosphate supplement. Plasma phosphate levels should be checked frequently to guide supplementation.
Vitamin D is important for bone mineralisation and many other physiological processes. In preterm neonates, all vitamin D must be obtained from dietary sources. Most infants receiving milk feeds will require some form of vitamin D supplementation, normally administered in a combination multivitamin product (e.g., Abidec). For preterm infants with symptomatic anaemia who are in neonatal intensive care units, iron supplementation is used infrequently because these babies will often receive blood transfusions[8]. However, breast-fed preterm infants will commonly be discharged home on oral iron supplements (e.g., Sytron) to prevent iron deficiency anaemia developing at home during the first year of life. Formula milk is manufactured with additional iron content and therefore ex-preterm babies discharged on formula feeds generally do not require supplementation. Sodium is required for consistent linear growth and preterm infants can benefit from sodium supplementation (either oral or intravenous)[3]. It is unusual for infants to be discharged from hospital with sodium supplements.
Neurological issues
Pain and sedation
Morphine is used for two main indications in the neonatal intensive care unit: as an analgesic and, sometimes, as a sedative for invasively ventilated infants.
Analgesia may be required for severe or sustained pain (in this case it is given by infusion) or for the treatment of short-term pain (in which case it is administered as bolus doses). In any circumstance, morphine should only be used when necessary and babies should be monitored for respiratory depression. Infants should be weaned from the drug as soon as possible to avoid development of tolerance.
Paracetamol can be used as an alternative to morphine for the treatment of short-term pain and has the advantage of not causing respiratory depression. Oral sucrose or breast milk can be given before a painful procedure — evidence shows that the baby will cry less during the procedure, although the mechanism of action for this is unclear. Suckling on a dummy can have the same effect. Oral sucrose can be used in preterm infants, though caution should be exercised in extremely preterm infants, those weighing less than 1.5kg and those who are not fully enterally fed. Preterm babies who are unwell or who have a history of necrotising enterocolitis should not receive sucrose analgesia. Sucrose is not recommended for babies older than one month because it has not been shown to be effective for these patients[3]. Other pharmacological considerations for sick neonates are outlined in Box 2.
Box 2. Pharmacological considerations for sick neonates
The following issues should be consideredwhen making decisions regarding drug treatment of preterm infants.
Excipients to avoid
Formulation of liquids usually requires more excipients than solid dosage forms. Adverse effects caused by these excipients are of particular concern in preterm, low-birthweight infants due to their immature hepatic and renal function.
Ethanol is commonly added to medicines as a solvent or preservative, or to aid skin penetration of topical products. Ethanol readily crosses the blood-brain barrier in children and can cause adverse effects[9]. One example is the commercially available phenobarbital elixir which contains 38% alcohol. All neonates should be administered alcohol-free medicines so a suitable product should be sought.
Benzyl alcohol should also be avoided in neonates because the metabolic pathway converting benzyl alcohol to hippuric acid can become saturated, leading to an accumulation of benzoic acid and a condition known as “gasping syndrome”[9].
Other excipients that should be avoidedinclude propylene glycol and parabens[9].
Lack of information
Most medicines used for neonates and preterm neonates are not licensed for such patients and are therefore used off-label. This means that, generally, there is a lack of data to support their use or guide dosing. The British National Formulary for Children is an excellent reference for medicines use in children, but it does not give sufficient information about drug use in neonates and preterm neonates. The Northern Neonatal Formulary (NNF) gives detailed monographs for medicines used in neonates, with information on pharmacology, drug doses, monitoring requirements and supply of such products. Some units may still use in-house guidelines and formularies as an alternative to the NNF.
Medicines supply
Due to the low bodyweight of infants seen in neonatal care, there can be practical problems with drug administration. Doses are often calculated per kilogram of body weight and pharmacists have an important role in discussing drug dilutions and sourcing lower-strength preparations to improve accuracy of administration. There is also a role for the pharmacy department in establishing central intravenous additive services (CIVAS). These supply the neonatal unit with drugs made up under aseptic conditions to reduce the risk of reconstitution and calculation errors, as well as contamination on the ward, and lower the chance of infection during administration.
Breast milk and maternal drugs
Breastmilk is the best feed a preterm infant can receive. Therefore it is vital to establish whether medicines a mother is taking are safe for use when feeding with breast milk, or whether they could affect the supply of breast milk. It is important to appreciate that most information regarding the use of drugs in lactation is derived from studies of full-term infants, who will have substantially different pharmacokinetics to infants born preterm.
Hypoxic brain injury
Hypoxic brain injury can occur if there is a decrease in the amount of oxygen supplied to an infant’s brain close to the time of birth, followed by secondary damage after reoxygenation. This can lead to severe lifelong disability or death[10].
No specific medicines have been shown to prevent the damage caused by perinatal hypoxia. However, therapeutic cooling has been shown to reduce the incidence of severe disability, improve survival without neurological impairment and improve psychomotor development index scores compared with standard care (i.e., no cooling)[11]. The process involves cooling the infant to between 33C and 34C to prevent the secondary damage that can occur during reoxygenation. Treatment is ideally started within six hours of birth and continued for 72 hours, after which time the infant is gradually rewarmed.
Seizures
Neonatal seizures can occur as a result of brain injury, hypoglycaemia or another metabolic cause. Generally, phenobarbital is the first-line anticonvulsant used in neonatal care, although it is not normally continued for longer-term maintenance treatment. If phenobarbital does not control seizures, phenytoin can be considered as an alternative or added to treatment (phenytoin is not used first line because of its non-linear pharmacokinetics). Pyridoxine or biotin can be given if inborn errors of metabolism are the suspected cause of seizures[3].
Patent ductus arteriosus
During pregnancy, the placenta supplies oxygen to the foetus; blood does not need to circulate via the lungs and, therefore, the circulation of a developing foetus contains a vessel connecting the pulmonary artery to the aortic arch (allowing most blood to bypass the lungs, see Figure). This is known as the ductus arteriosus. During pregnancy, the ductus arteriosus is kept patent by circulating prostaglandins. In the seven to 14 days after delivery various chemical triggers (including higher levels of circulating oxygen from the lungs) close the ductus arteriosus functionally (by constriction) then permanently(by scarring and fibrosis). Once this process is complete, blood cannot bypass the lungs.
Figure. Foetal cardiopulmonary circulation
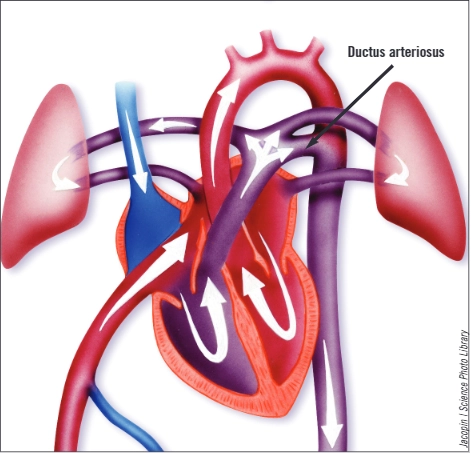
In very preterm infants, the mechanisms that close the ductus arteriosus are not fully mature and patency of the ductus is common. If the ductus arteriosus is large, allowing blood from the systemic circulation to be shunted into the pulmonary circulation, it can cause problems with ventilation, tolerance of enteral feeding and growth.
A patent ductus arteriosus can be closed medically, using non-steroidal anti-inflammatory drugs that promote ductal closure (commonly indometacin or ibuprofen). However, renal impairment can limit their use. Failure of medical treatment can prompt the need for surgical intervention.
Necrotising enterocolitis
Necrotising enterocolitis is an inflammatory disease of the gastrointestinal tract, characterised by ulceration and, occasionally, perforation of the bowel. It is the most common gastrointestinal emergency seen in neonates and has a mortality rate of 20–40%. Management depends on the stage of the disease, with medical management preceding and supporting any surgical interventions.
Antibiotics should be started as soon as necrotising enterocolitis is suspected. Use of metronidazole in combination with two broad-spectrum antibiotics is common; treatment is continued for seven to 14 days.
Withholding enteral feeds, to allow the bowel to rest, is vital. Total parenteral nutrition should be started to ensure nutritional needs, which are increased during infection or stress, are met. Respiratory and cardiac support may be needed if an infant is systemically unwell as a result of the necrotising enterocolitis. Short bowel syndrome is a rare and sometimes fatal consequence of extensive resection to treat necrotising enterocolitis[12].
Neonatal sepsis
Neonatal sepsis can be categorised as early- or late-onset. Early-onset sepsis syndrome occurs within the first four days of life. Organisms causing early-onset sepsis are acquired from the mother, commonly Gram-negative organisms (particularly Escherichia coli) and group B streptococci.
Late-onset sepsis syndrome occurs between days 4 and 90 of life, and causative organisms are acquired from the environment (e.g., coagulase-negative staphylococci from the infant’s skin infiltrating the blood stream via indwelling vascular devices).
Treatment of neonatal bacterial infections must be prompt and appropriate for the organisms being targeted — treatment decisions should be based on results of blood culture and sensitivity testing to reduce the risk of antibiotic resistance. Nevertheless, antibiotics are often started empirically while awaiting cultures, and then treatment is continued, stopped or changed once a positive diagnosis and sensitivities are known. High-risk preterm neonates may require prophylactic antifungal treatment, either with nystatin or fluconazole, because this has been shown to reduce the incidence of invasive fungal sepsis in the context of empirical antibacterial prescribing[3].
Retinopathy of prematurity
Retinopathy of prematurity is a retinal vasoproliferative disease that affects premature infants and can lead to retinal detachment and blindness. It is caused by early cessation of normal retinal vessel growth and the proliferation of abnormal vessels[13]. Infants born very preterm are regularly screened for abnormal vascularisation. If required, treatment involves laser therapy. There are case reports of the use of intraocular bevacizumab for salvage treatment of progressive retinopathy of prematurity[14]. This is an unlicensed indication and its use is not commonplace.
Summary
Management of preterm infants in neonatal intensive care units can be broadly categorised as supportive or reactive. Supportive care aims to maintain normal homeostatic functioning through respiratory, cardiac and nutritional support. Reactive care involves treatment of a neonate’s medical complications, such as sepsis, patent ductus arteriosus, necrotising enterocolitis, retinopathy of prematurity or neurological issues.
Use of medicines in preterm infants can be complicated, since there is often little evidence for their use. Practical challenges with formulation and administration are also common.
References
- Nkadi PO, Merritt TA, Pillers DA. An overview of pulmonary surfactant in the neonate: genetics, metabolism, and the role of surfactant in health and disease. Molecular Genetics and Metabolism 2009;97:95–101.
- Engle WA. Surfactant-replacement therapy for respiratory distress in the preterm and term neonate. Pediatrics 2008;121:419–32.
- Hey E (ed). Neonatal formulary: drug use in pregnancy and the first year of life, 6th edition. London: BMJ Books; 2011.
- Shinwell ES, Karplus M, Reich D, et al. Early postnatal dexamethasone treatment and increased incidence of cerebral palsy. Archives of Diseases in Childhood Fetal and Neonatal Edition 2000;83:F177–81.
- Rademaker KJ, de Vries WB. Long-term effects of neonatal hydrocortisone treatment for chronic lung disease on the developing brain and heart. Seminars in Fetal and Neonatal Medicine 2009;14:171–7.
- Schmidt B, Roberts RS, Davis P, et al. Caffeine therapy for apnea of prematurity. New England Journal of Medicine 2006;354:2112–21.
- Osborn DA, Paradisis M, Evans NJ. The effect of inotropes on morbidity and mortality in preterm infants with low systemic or organ blood flow. Cochrane Database of Systematic Reviews 2007; issue 1.
- Embleton ND. Enteral nutrition for preterm infants: translating ESPGHAN guidelines into practice. Infant 2011;7:187–90.
- Costello I, Long P, Wong IK, et al. Paediatric drug handling. London:Pharmaceutical Press; 2007.
- National Institute for Health and Clinical Excellence. Therapeutic hypothermia with intracorporeal temperature monitoring for hypoxic perinatal brain injury. May 2010. Available at: www.nice.org.uk/ipg347 (accessed 1May 2012).
- Jacobs SE, Hunt R, Tarnow-Mordi WO, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews 2007; issue 4.
- Manaf A, Morabito A. Understanding the management of necrotising enterocolitis. British Journal of Clinical Pharmacy 2011;3:15–7.
- Lee J, Dammann O. Perinatal infection, inflammation and retinopathy of prematurity. Seminars in Fetal and Neonatal Medicine 2012;17:26–9.
- Dani C, Frosini S, Fortunato P, et al. Intravitreal bevacizumab for retinopathy of prematurity as first line or rescue therapy with focal laser treatment. A case series. Journal of Maternal-Fetal and Neonatal Medicine 17 April 2012 [online ahead of print] (accessed 1 May 2012).


