This content was published in 2010. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
Summary
Treatment of rheumatoid arthritis has developed substantially over recent years, reducing the likelihood of deformity and disability. Several of the newer biologic medicines, which act against tumour necrosis factor and other specific targets in the inflammatory response, are now approved by the National Institute for Health and Clinical Excellence and/or the Scottish Medicines Consortium. Disease-modifying antirheumatic drugs and corticosteroids are also used.
Despite these advances many patients still struggle with the condition because their therapy has not been optimised or because of intolerance to drugs. Pharmacists can help patients manage their medicines, advise on side effects and, with specialist training, perform prescribing and monitoring roles.
Early intervention is crucial for people with rheumatoid arthritis, because there is a window of opportunity in which to prevent joint damage and reduce the chance of subsequent deformity and vascular effects, such as myocardial infarction and cerebrovascular accident.1 Even when a clear diagnosis of rheumatoid arthritis has not been made, but there is definite evidence of an inflammatory arthritis, it is crucial that introduction of effective treatment is not delayed.2
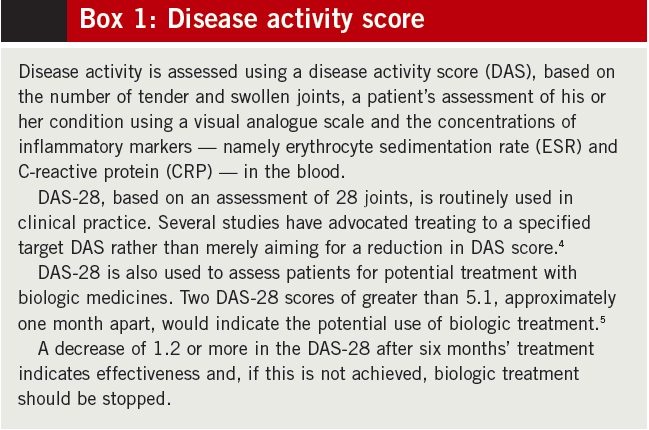
Pharmacological treatments play a key role in managing both the early and the later stages of RA. The National Institute for Health and Clinical Excellence’s clinical guideline on RA outlines the current treatment approach.3 Not only is early use of disease-modifying antirheumatic drugs (DMARDs) encouraged, but combinations of DMARDs are advocated following diagnosis, alongside use of injected or oral steroids. There can often be a fine balance between achieving disease control (assessed using disease activity score — see Box 1) and minimising side effects.
Disease-modifying drugs
Whereas most DMARDs have been available for many years, it is only relatively recently that the importance of adequate dosing and early intervention (ie, within three months of the start of persistent symptoms) has been understood. The NICE RA clinical guideline recommends combination treatment for newly diagnosed RA, including methotrexate and at least one other DMARD, unless a patient is pregnant or has other relevant conditions. When particular drugs cannot be tolerated, any combination of DMARDs can be tried, the balance always being between obtaining a desirable therapeutic effect and minimising toxicity.
Methotrexate
Up to 25mg of methotrexate, either as monotherapy or in combination, can be given each week. Doses can be achieved by rapid escalation, for example starting at 7.5mg or 10mg and increasing by 2.5mg per week. Combination DMARD treatments include “triple therapy” with methotrexate, sulfasalazine and hydroxychloroquine, and methotrexate plus leflunomide. Despite evidence for their effectiveness in RA, the mechanism of action of DMARDs is not fully understood. Methotrexate is known to inhibit the activation of T lymphocytes, as well as reduce folate synthesis. It is believed that such actions are complementary to inhibition of tumour necrosis factor (TNF), therefore explaining the benefits of combining methotrexate with anti-TNF medicines.
Sulfasalazine
Doses of 2–3g per day of sulfasalazine enteric-coated tablets, split over two or three mealtimes, can be given. When starting treatment, gradual dosing is used to improve tolerance. An initial dose of 500mg is given daily for one week, followed by 500mg twice a day for the second week, then 1g in the morning and 500mg in the evening for the third week and then 1g twice a day thereafter. Sulfasalazine can reduce concentrations of interleukin-1 and TNF-alpha, as well as inhibiting T cell activation and inducing T cell death.
Hydroxychloroquine
Inhibiting the antigen processing function of macrophages and other antigen-presenting cells (such as B cells) is believed to be the mode of action of hydroxychloroquine. Its usual dose is 200mg twice daily, usually in combination with other DMARDs.
Leflunomide
Licensed for RA in the UK in 2000, leflunomide is a newer DMARD. It affects the uridine synthesis pathway, which is important in T cell proliferation. Although its extremely long half-life means it has licensed loading doses of 100mg daily for three days, in practice such doses can cause severe diarrhoea and abdominal discomfort and, thus, treatment is usually commenced at the usual daily dose of 10mg or 20mg.
Sodium aurothiomalate
First used in the 1920s, sodium aurothiomalate (gold) is still used in clinical practice and is thought to act at several stages of the inflammatory pathway, including impairment of T cell activation, and inhibition of TNF-alpha, interleukin-1 and interleukin-12. It is administered by intramuscular (IM) injection.
Corticosteroids
Particularly in the early stages and in controlling “flares” of disease, corticosteroids have an important place in bringing RA under control. Although they can be prescribed orally, it is preferable to administer one-off IM injections when several joints are affected or intraarticular (IA) injections for specific joints.
Although there are limited data on the efficacy of corticosteroids in RA, they are used widely in practice. There is often a mistaken belief that IA injections can be administered up to three times a year only; however, there is some evidence that they should be administered as frequently as required to suppress inflammation and prevent joint damage.6 Nonetheless, regular injections over a prolonged period would indicate uncontrolled RA, which may necessitate adjustment or alteration of DMARD treatment or progression to the use of biologic therapies.
Biologics
Although the earlier and more aggressive use of DMARDs and corticosteroids has markedly improved RA treatment, the introduction of biologic medicines has been a major breakthrough. Development of biologics relied on an enhanced understanding of the role of cytokines in inflammatory pathways, as well as advances in recombinant monoclonal antibody and other technologies.
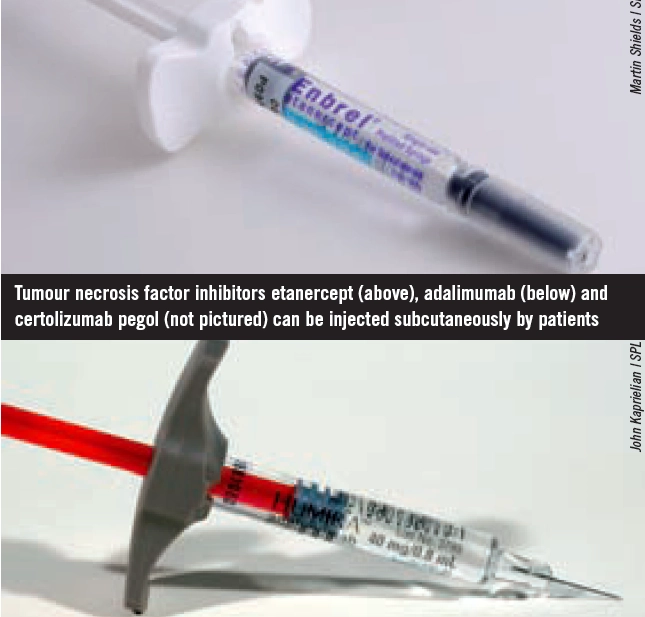
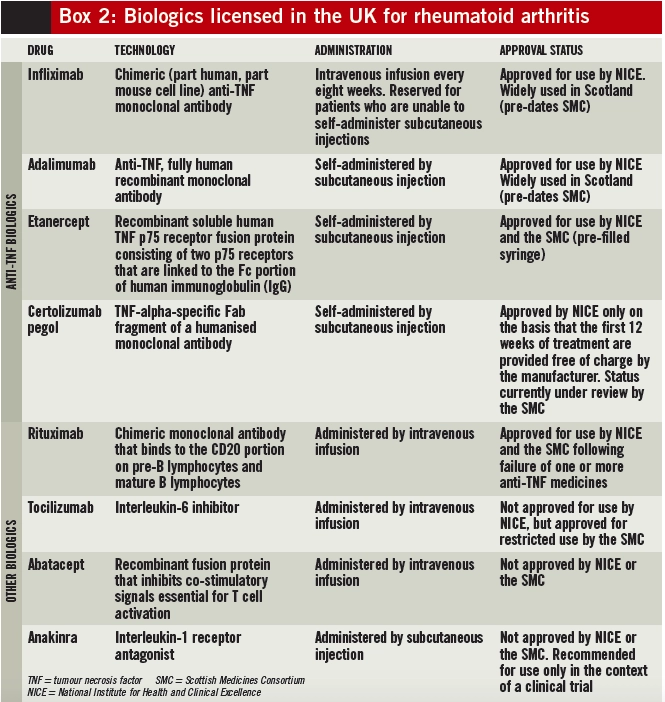
Infliximab, adalimumab, etanercept and certolizumab pegol are anti-TNF medicines. They neutralise TNFalpha (an important inflammatory mediator in RA, produced by B lymphocytes directly and by macrophages activated by T cells) in both the synovium and blood. Rituximab, tocilizumab, abatacept and anakinra act at different sites in the inflammatory pathway. Several biologics have been approved for use by NICE and the Scottish Medicines Consortium (see Box 2).
It is important to understand that cytokine expression can differ greatly between patients, hence the hypothesis that some patients will respond to certain treatments while others may not.7 Although there is some evidence that testing positive for serum rheumatoid factor (RF) and antibodies against citrullinated peptides (anti-CCP) might indicate a greater response to rituximab,8 it is important that a stepwise, methodical approach to treatment is adopted for all patients.
Patients have previously been able to switch from one anti-TNF medicine to another if the first one has been ineffective (or they have been intolerant to it). However, NICE has recently issued provisional guidance, currently under consultation, recommending that patients who have not responded to one anti-TNF agent should not be switched to an alternative, but can be considered for treatment with rituximab. Final guidance on the matter is due to be issued by NICE in summer 2010.
Minimising adverse effects
Serious side effects are associated with many of the medicines used to treat RA. Intolerance to medicines can also result in reduced patient outcomes.
DMARDs
Before starting DMARD treatment, it is important for clinicians to check several baseline parameters so that toxicity can be more easily identified and causality can be linked to the therapy used.9 For example, methotrexate treatment can cause interstitial pneumonitis; however, RA itself can cause lung disease, so it is necessary that a chest X-ray has been undertaken in the six months before treatment starts. Many centres also undertake lung function tests to provide a baseline measurement and allow easy comparison with subsequent measurements and this should be routine for patients with existing lung disease and for smokers. Liver function and blood tests are also needed and renal function (ie, urea and electrolytes) should be monitored since methotrexate is excreted by the kidneys.
Folic acid (5–30mg per week)10 is given on one or more of the days that methotrexate is not taken. Folic acid may decrease the gastrointestinal adverse effects and liver toxicity of methotrexate, as well as the incidence of mouth ulcers and stomatitis. Self-administration of methotrexate by subcutaneous injection can also reduce gastrointestinal intolerance (and increase bioavailability).
Whether to stop methotrexate, or other DMARDs, when an infection is suspected in a patient is an important consideration. It has been proposed that mild viral infections, such as those of the upper respiratory tract, do not require cessation of methotrexate, whereas bacterial infections requiring treatment with antibiotics will require temporary cessation. For severe infections methotrexate should not be reintroduced until inflammatory markers have returned to baseline levels and clinical symptoms have resolved.11
Leflunomide, like methotrexate, is associated with interstitial pneumonitis and patients should be advised to stop taking the medicine and seek advice if they become acutely short of breath. Leflunomide has a long half-life and, in the case of serious infection, stopping the drug will not be sufficient; it must also be “washed out” using colestyramine, which prevents enterohepatic recycling.
Full blood count, liver function tests, renal function tests and urine analysis should precede sodium aurothiomalate injections.
Visual acuity should be checked annually in patients taking hydroxychloroquine because of the potential for retinal toxicity.
Biologics
The key concern of treatment with TNFalpha inhibitors is the risk of infection, since TNF-alpha is inherent to the immune response. Reactivation of latent tuberculosis is a particular concern, and all patients should be screened for tuberculosis before commencing anti-TNF treatment.12
Although data are limited, the risk of infection appears to be less with other biologics (eg, tocilizumab, rituximab). Rituximab has been associated with three cases of progressive multifocal leukoencephalopathy in the US. This is a serious neurological complication (with two cases proving fatal) although incidence is rare.
All potential adverse effects and benefits of biologic treatment must be discussed with patients as part of the process for obtaining informed consent.
NSAIDs
Patients with inflammatory arthritis often require symptomatic treatment with non-steroidal antiinflammatory drugs (NSAIDs), even if only in the short term. Their potential gastrointestinal toxicity is well documented, but recent evidence suggests that all NSAIDs, with the exception of naproxen, carry an increased risk of cardiovascular complications. This has implications for RA patients, particularly with longerterm treatment, since they already have an inherently increased cardiovascular risk. These issues should be considered and discussed with patients. Those prescribed NSAIDs long term should also receive a proton pump inhibitor.3
Corticosteroids
The potential toxicity of corticosteroids is well documented and regular oral treatment should be avoided. Long-term treatment with corticosteroids reduces bone density and this is a particular issue because RA itself is associated with fractures. Intermittent use of IM or IA injections has not been associated with increases in fractures and can actually reduce bone loss by preventing erosion. Nevertheless, patients should be counselled regarding potential risks of psychotic reactions with higher (IM) doses and the risk of infection following IA injections (of 1 in 10,000).
Surgical options
Recent advances in drug treatment have reduced the requirement for surgical intervention, such as arthroplasty and arthrodesis. However, surgery remains an option, particularly for patients with localised and progressive deformity.3
Many rheumatology centres run combined clinics with orthopaedic surgeons. This approach affords patients the opportunity to discuss possible surgical options within the context of the overall management of their condition, instead of viewing surgery as a last resort.
Multidisciplinary care
The management of RA is underpinned by a collaborative approach from a range of healthcare professionals, all playing a part in the ongoing education of patients and each other.
Specialist nurses generally act as the first point of contact for patients and support them with the physical, social and emotional aspects of the condition. Many nurses will also perform the monitoring required for DMARD and biologic treatments.
Occupational therapists undertake home assessments and provide suitable modifications of, for example, bathroom facilities and kitchen utensils. They can provide ready-made and bespoke splints and supports for certain joints.
Physiotherapists perform specific assessments of physical capability and can provide management through exercise programmes. Some will also provide IA corticosteroid injections.
Podiatrists play an important role, since 75% of patients with the condition have difficulty in walking because of problems with joints in their feet. They can provide various shoe inserts and supports and specialist podiatrists also undertake foot surgery.
Specialist pharmacists
While the multidisciplinary rheumatology team will usually have a pharmacist, even if only as a named “link”, several pharmacists in the UK have developed specialist roles in this area. These involve the direct management of patients in outpatient clinics, prescribing DMARDs and administering IA injections, as well as undertaking the monitoring required for treatment with DMARDs and biologics.13
Community pharmacists
Community pharmacists will see many patients with RA (as well as other inflammatory conditions that have some common characteristics, eg, psoriatic arthritis). As part of the wider multidisciplinary team, they can offer practical and emotional support in addition to providing expertise in the management of patients’ medicines (eg, identifying side effects, barriers to adherence, etc). Specifically, community pharmacists should ensure that patients receiving methotrexate have monitoring booklets that reflect their current dose, in accordance with National Patient Safety Agency requirements.
Community pharmacist can provide reassurance to patients to continue with DMARD treatment despite the presence of a mild viral respiratory illness but also to be vigilant for underlying infection in patients receiving anti-TNF treatment. For the latter patients it is important to note that, although they will usually be receiving a DMARD concomitantly, their anti-TNF therapy is unlikely to be recorded on their repeat prescription and may not even be recorded on a GP medication record. If there is any doubt regarding the presence of infection, patients should be encouraged to contact their GP or their specialist rheumatology nurse or pharmacist.
Always keeping in mind the potential vascular complications of RA, community pharmacists are also in a position to review patients’ entire drug regimens and to offer lifestyle advice, such as taking regular exercise, moderating alcohol consumption and stopping smoking.
Specialist group
For pharmacists interested in joining the UK Rheumatology Pharmacists Association, further information is available at http://sites.google.com/ site/ukrheuphar.
References
- Klareskog L, Anca Irinel C, Paget S. Rheumatoid arthritis. Lancet 2009;373:659–72.
- Combe B, Landewé R, C Lukas, et al. EULAR recommendations for the management of early arthritis: Report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Annals of the Rheumatic Diseases 2007;66:34–45.
- National Institute for Health and Clinical Excellence. Rheumatoid arthritis: the management of rheumatoid arthritis in adults. February 2009. www.nice.org.uk/cg79 (accessed 12 April 2010).
- Goekoop-Ruiterman YPM, DeVries-Bouwstra JK, Allart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): A randomized, controlled trial. Arthritis & Rheumatism 2005;52:3381–90.
- National Institute for Health and Clinical Excellence. Adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis. October 2007. www.nice.org.uk/ta130 (accessed 12 April 2010).
- Grigor C, Capell H, Stirling A, et al. Effect of a treatment strategy of tight control for rheumatoid arthritis (the TICORA study): a single-blind randomised controlled trial. Lancet 2004;364:263–9.
- Ulfgren AK, Grondal L, Lindblad S, et al. Interindividual and intraarticular variation of proinflammatory cytokines in patients with rheumatoid arthritis: potential implications for treatment. Annals of the Rheumatic Diseases 2000;59:439–47.
- Teng YK, Levarht EW, Hashemi M, et al. Immunohistochemical analysis as a means to predict responsiveness to rituximab treatment. Arthritis & Rheumatism 2007;56:3909–18.
- British Society for Rheumatology. Guideline for disease-modifying antirheumatic drug (DMARD) therapy in consultation with the British Association of Dermatologists. 2008. www.rheumatology.org.uk (accessed 12 April 2010).
- Bramley D. Medicines Q&A: What is the dose of folic acid to use with methotrexate therapy for rheumatoid arthritis? www.nelm.nhs.uk (accessed 12 April 2010).
- McLean-Tooke A, Aldridge C, Waugh S, et al. Methotrexate, rheumatoid arthritis and infection risk — what is the evidence? Rheumatology 2009;48:867–71.
- British Thoracic Society. Recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-α treatment. Thorax 2005;60:800–5.
- Copeland R. The role of the specialist pharmacist in rheumatology. Hospital Pharmacist 2008;15:215–16.