This content was published in 2005. We do not recommend that you make any clinical decisions based on this information without first ensuring you have checked the latest guidance.
Arrhythmias develop as a result of either altered impulse generation or impulse conduction in the myocardium. Treatment options are pharmacological (eg, amiodarone, lidocaine, atenolol), ablative (destruction of the malfunctioning tissue) or electrical to correct the rhythm.
Drug therapy
Anti-arrhythmic drug therapy is used to control the frequency and severity of arrhythmias, with the aim of maintaining sinus rhythm where possible. The drugs can be grouped according to their electrophysiological effects at a cellular level, using a system known as the Vaughan Williams classification. Alternatively, drug therapies can be divided according to their main sites of action within the heart.
Vaughan Williams classification
The flow of sodium, calcium and potassium ions is key to the process of myocardial depolarisation. Five clear phases can be identified during the process of depolarisation and repolarisation (see Figure 1). The phases are:
- Phase 0: Fast sodium channels are responsible for initial rapid depolarisation
- Phase 1: Early fast repolarisation
- Phase 2: Prolonged depolarisation “plateau” due to slow calcium influx
- Phase 3: Repolariastion due to closing of the calcium channels and potassium efflux
- Phase 4: Resting membrane potential is restored.
All anti-arrhythmic drugs act by altering the movement of electrolytes within the electrical conduction pathways of the myocardium. The Vaughan Williams classification system groups drugs according to their ability to block the movement of one or more of these ions across the myocardial cell membrane.
Class I
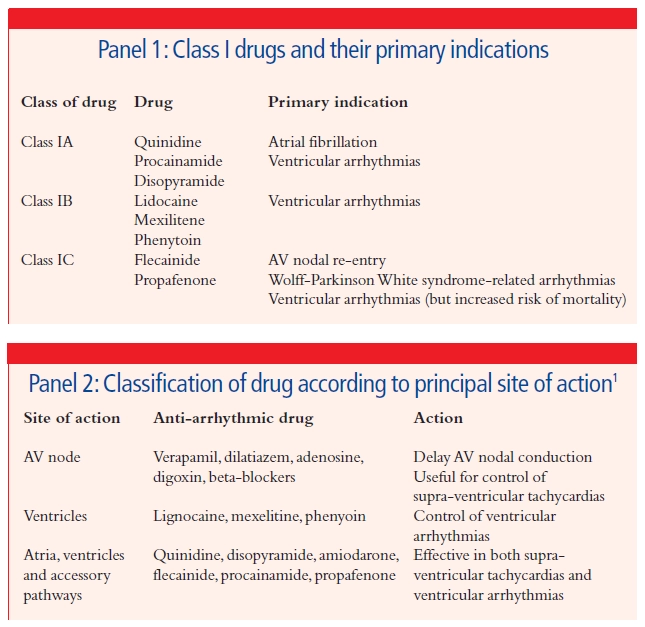
Class I drugs act by blocking the fast sodium channels and therefore delay the rise in phase 0 of the action potential. This group can be further subdivided (see Panel 1) into: class A drugs which increase the duration of the action potential; class IB, which shorten the action potential; and class IC, which have no effect on action potential duration. These effects can be assessed by measurement of the QT interval on the electrocardiogram (ECG), which is an indication of action potential duration.
Class II
Class II drugs act by modifying the sympathetic stimulation of heart rate by blocking the beta-adrenoceptors. Although, these drugs do not affect the action potential in most myocardial cells, they increase the time taken to reach the threshold potential during phase 4 in the specialised pacemaker cells, hence slowing sinoatrial (SA) nodal and atrioventricular (AV) nodal firing.
Class II drugs are more commonly known as beta- blockers and include atenolol, bisoprolol, carvedilol, metoprolol and propranolol, among others. In the management of arrhythmias, beta-blockers are commonly used to slow the heart rate in patients with atrial fibrillation (AF) or atrial flutter. These agents do not return the heart to sinus rhythm (cardioversion) but control the ventricular rate and hence reduce the symptoms associated with the arrhythmia.
Class III
Class III agents act by blocking the potassium channels and therefore prolonging the plateau of phase 2, delaying repolarisation, and increasing the refractory period and preventing further excitation. Class III drugs include amiodarone, sotalol and bretylium. Primary indications for class II agents are AF atrial flutter and ventricular tachycardias. In AF and atrial flutter, these agents can pharmacologically return the heart to sinus rhythm as well as improve rate control. Amiodarone, in particular, is useful to increase the likelihood of successful direct current (DC) cardioversion (discussed later) in patients who failed at the first attempt.
Class IV
Class IV agents block the movement of calcium ions during phase 2 (plateau phase) of the action potential. In addition, calcium movement is the primary determinant of depolarisation in SA and AV nodal pacemaker cells and the nodes are therefore particularly sensitive to the effects of calcium channel blockers. Only the non-dihydropyridine calcium channel blockers have effects on conduction (eg, diltiazem and verapamil). This group is useful in the management of AF or atrial flutter, atrial arrhythmias due to increased automaticity and AV nodal re-entry tachycardias.
Other drugs
Some drugs cannot be classified according to this system. Digoxin increases AV nodal delay and is useful to control the ventricular response to AF and atrial flutter. It does not increase the likelihood of cardioversion to sinus rhythm, but can reduce the symptoms associated with rapid ventricular rates in this condition (eg, syncope [ie, fainting], breathlessness and lethargy).
Adenosine blocks conduction across the AV node and is used in the acute situation to terminate AV nodal re-entry tachycardias and can be used to determine the underlying disease in supra-ventricular tachycardias. However, it must not be given if there is a suspicion of ventricular tachycardia (VT), because of an increased risk of severe hypotension.
Classification according to site of action
A more simple classification system used in clinical practice is to consider the principal site or sites of action of anti-arrhythmic drugs (see Panel 2). Once the cause of an arrhythmia has been determined, a suitable first-line anti-arrhythmic can then be chosen to treat the arrhytmia, but also to suit the individual patient based on age, co-morbidities, lifestyle and likelihood of compliance.
Pitfalls of anti-arrhythmic drugs
When recommending, prescribing or monitoring anti-arrhythmic drugs, pharmacists should be aware of their drawbacks.
Efficacy
It is important to acknowledge that the efficacy of anti-arrhythmic therapy is limited. Although the choice of agent for a specific arrhythmia may be based on a sound scientific theory, it may still fail to work in an unsatisfactorily high proportion of patients. Choice of drug and dose can therefore become a system of trial and error and may well be a laborious process. The lack of clinical effectiveness has led to the continued search for different methods of managing arrhythmias and the development of non-drug options such as radiofrequency (RF) ablation, pacing techniques and internal cardioversion defibrillators (ICDs) (all described later).
Adverse effects
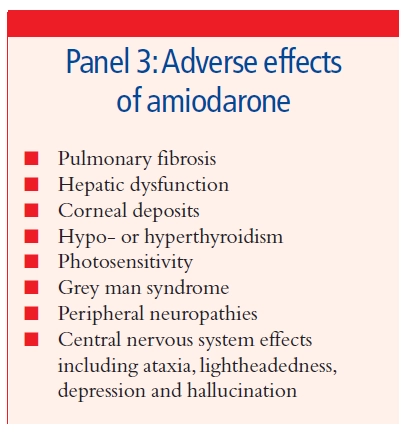
The search for a drug that controls the arrhythmia is also confounded by the high incidence of adverse effects with this group of drugs which can limit patient acceptability. Adverse effects vary depending on the particular agent, but can include gastrointestinal disturbance (diarrhoea, nausea, vomiting), hypotension, heart failure, central nervous system effects (such as tremor, headache, depression, hallucination, night- mares, psychosis seizures), visual disturbance and hypersensitivity reactions. Amiodarone, now widely prescribed in the UK for a variety of arrhythmias, is particularly toxic in the long-term (see Panel 3). Close monitoring of all patients pre- scribed anti-arrhythmic therapy is therefore essential.
Safety
A major problem with the use of anti-arrhythmic agents is their propensity to be pro-arrhythmic. In others words, through attempting to treat an arrhythmia pharmacologically, the condition can paradoxically be exacerbated in some patients, or new arrhythmias may be generated. This is a particular problem with class I and class II drugs, affecting up to 20 per cent of patients. These pro-arrhythmic effects can, in some cases, increase the risk of mortality. Two specific pro-arrhythmic effects are well-recognised: torsades de pointes and monomorphic VT. The latter was first described following publication of data from the Cardiac Arrhythmia Suppression Trial (CAST). Treatment with flecainide (a class III agent) was found to increase the risk of patients developing a monomorphic, sustained VT and was associated with a small increase in mortality. Patients at greatest risk are those with previous sus-tained VT, ischaemic heart disease or left ventricular systolic dysfunction.
Aside from the evidence from CAST, there are few data to define clearly the impact of anti-arrhythmic therapy on mortality. Evidence suggests that beta-blockers and possibly amiodarone reduce mortality, which may explain their relative popularity compared with other options. Limited evidence suggests that disopyramide, mexelitine and quinidine may increase long-term mortality, but this is not conclusive. With this is mind, the decision to use class I and class II agent particularly must be made with due consideration of both the potential benefits and the associated risks.
Non-drug interventions
Non-drug interventions are being used increasingly to treat arrhythmias and they are reviewed below.
Radiofrequency ablation
Radiofrequency ablation represents a major advance in the management of arrhythmias, particularly supra-ventricular tachycardias. Success rates are high (approximately 90 per cent) with a low complication rate. The procedure destroys the aberrant conduction pathways present in certain arrhythmias, preventing future arrhythmias and obviating the need for long-term anti-arrhythmic drugs.
Electrophysiological studies must be carried out to allow mapping of electrical activity within the heart and the location of re-entry circuits or foci responsible for generating arrhythmias before RF ablation can be performed. A catheter with an electrode sited at the tip is then guided to the appropriate point in the myocardium. RF energy is transmitted locally to destroy the tissues and disrupt the conduction pathway. The procedure is usually performed under light sedation in adults and patients can quickly return to their normal activities.
RF ablation is frequently the first-line option in the management of arrhythmias such as AV nodal re-entry tachycardia or AV re-entry tachycardia including Wolff- Parkinson White syndrome, AF atrial flutter and ventricular tachycardia.
Direct current cardioversion
Cardioversion refers to the process of restoring the heart’s normal rhythm, usually in patients with atrial flutter or AF This can be done chemically using drugs such as amiodarone, flecainide, sotalol or verapamil. It is more commonly achieved by the application of a controlled electric shock across the chest wall to override disordered conduction and allow the sinus node to regain control of heart rate. The patient is briefly anaesthetised while the shock is delivered. At first attempt, DC cardioversion is successful in up to 80 per cent of cases. A second attempt at DC cardioversion may be made following the initiation of anti-arrhythmic medicines.
DC cardioversion is less successful in older patients, long-standing AF patients with mitral valve disease and those with cardiomyopathies. Anti-arrhythmics may be required long-term to suppress the frequency of episodes in patients persistently reverting to AF or atrial flutter. AF or atrial flutter unresponsive to DC cardioversion is termed persistent and is managed chronically with drug therapy to control ventricular rate.
In patients with chronic AF cardioversion to sinus rhythm increases the risk of embolus formation, if there is a thrombus present in the atria. Patients in AF for more than 48 hours must be fully anticoagulated for four weeks pre-cardioversion to reduce the risk of stroke or other thromboembolic events. Co-ordinated atrial activity may not resume for two weeks following cardioversion even if sinus rhythm is apparent on the ECG and for this reason anticoagulation should continue for a further four weeks. As an alternative, a trans-oesophageal echocardiogram may be undertaken pre-procedure to visualise the left atrium and exclude the presence of a thrombus.
Defibrillation
Defibrillation is performed to correct life-threatening fibrillations of the heart, which could result in cardiac arrest. It should be performed immediately after identifying that the patient is experiencing a cardiac emergency, has no pulse and is unresponsive. The process is similar to DC cardioversion, using the delivery of an electric shock to the myocardium via the chest wall. The success of defibrillation depends on the speed with which it is delivered, with a 10 per cent reduction in successful outcome with every minute that ventricular fibrillation persists. Cardiopulmonary resuscitation should be performed before and between the delivery of shocks to ensure a minimum cardiac out- put to maintain life and reduce cerebral damage.
Pacemakers
Pacing is employed on a temporary or permanent basis to correct bradycardia. The most common indication for temporary pacing is post-myocardial infarction (MI) with the development of new bundle branch block with first degree heart block or symptomatic bradycardia in anterior MI or second-degree AV block in an anterior MI.
In recent years, the role of temporary pacing has diminished because of improved reperfusion strategies post-MI and_ better risk stratification systems to ensure early per- pacing appropriate. Permanent pacemaker systems are implanted to correct a variety of different bradycardia. Typical indications are complete heart block, sick sinus syndrome, symptomatic degree AV block or symptomatic bradycardia. Permanent pacemakers are also required in some patients post-RF ablation, for example following AV nodal ablation for persistent AP with a rapid ventricular rate.
Permanent pacemaker systems are implanted in a skin “pocket” below the col- lar bone. Leads are inserted via a local vein into the heart and positioned under X-ray guidance. The leads can be used to sense the electrical activity within the heart to assess the underlying rate and rhythm. If appropriate, they can deliver small electrical impulses to the myocardial tissues to generate an action potential and stimulate myocardial contraction. Leads can be placed in both atria and ventricles and a number of different treatment algorithms (including AF termination algorithms) programmed depending on the intrinsic cardiac rhythm.
Internal cardioversion defibrillators
Internal cardioversion defibrillators (ICDs) are implanted in high-risk patients to pre- vent sudden cardiac death due to malignant ventricular tachycardias. National Institute for Clinical Excellence guidance recommends that ICDs should be considered in the following circumstances:
- For secondary prevention in patients with cardiac arrest due to VT or ventricular fibrillations, or spontaneous sustained VT with haemodynamic compromise (including syncope), or sustained VT in presence of left ventricular systolic dysfunction
- For primary prevention post-MI in patients with non-sustained VT on 24 hour tape and positive VT stimulation tests and left ventricular systolic dysfunction
The devices are implanted and monitor the cardiac rate and rhythm in a similar manner to permanent pacemakers. However, on sensing a VT, the device will initially deliver anti-tachycardia pacing impulses at a rapid rate (faster than the arrhythmia) to try to regain control of cardiac rhythm and then slow the heart rate down in a controlled manner. If this fails, the device will discharge an internal electric shock (at a higher volt- age than for DC cardioversion) to terminate the arrhythmia and return the heart to sinus rhythm. This can be an unpleasant experience for the patient, and additional anti-arrthythmic drugs may be required to ensure episodes occur infrequently and to reduce the number of inappropriate shocks. The role of ICDs in clinical practice is expanding with the publication of favourable trial data comparing them to pharmacological interventions to manage arrhythmias. Their widespread use is primarily limited by the cost per device (approximately £15,000 at the current time).
Summary
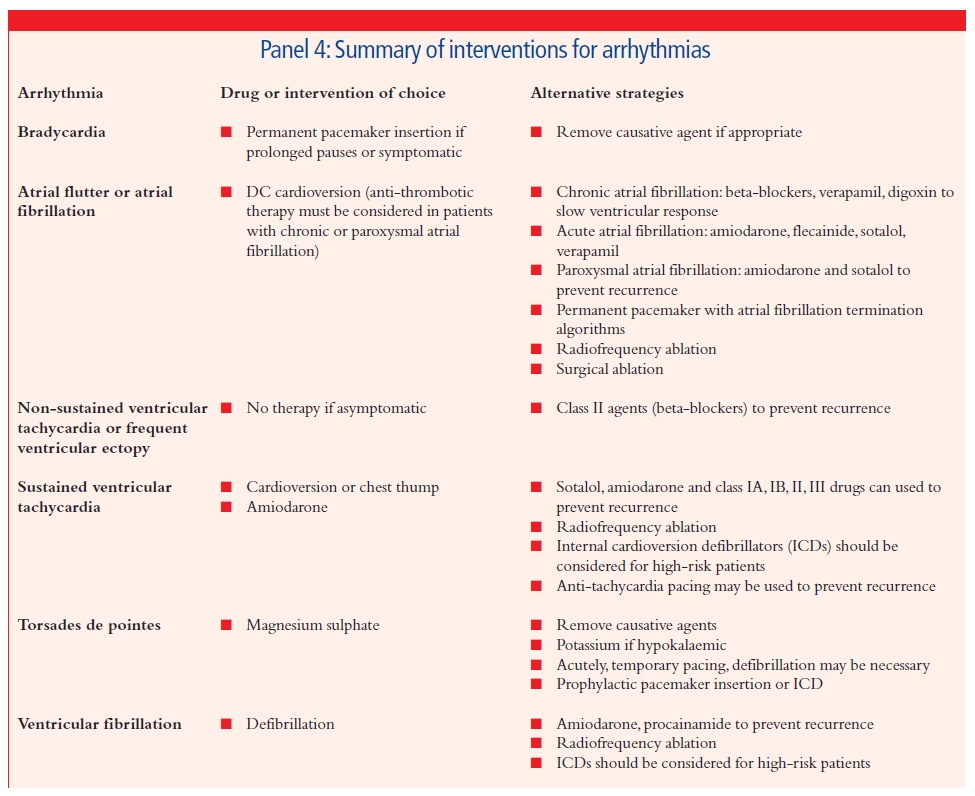
The management of arrhythmias is a complex business. It is important to look first for any underlying cause and correct this if possible. Treatment decisions for individual patients should then be driven by the under- lying risk associated with the arrhythmia, degree of symptoms experienced, potential efficacy of management strategies and the associated risk of adverse effects. The role of drugs has diminished somewhat over the past few years as interventional procedures and device technology have progressed. Panel 4 summarises the first-line and alter- native therapies for the management of arrhythmias commonly seen in clinical practice. As has already been mentioned, first-line drug therapy may not be effective in all patients and drug intolerance is com- mon. Finding an appropriate management strategy is therefore often a matter of trial and error. In many cases a combination of drug therapy and an implantable device or ablation procedure may be appropriate.
References
- Bennet DH. Cardiac arrhythmias 5th ed. Cambridge: Butterworth Heinemann; 1997.
- Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, et al. Mortality and morbidity in patients receiving encainide, flecainide or placebo (CAST). New England Journal of Medicine 1991;324:781-8.
- National Institute for Clinical Excellence. Guidance on the use of implantable cardiovertor defibrillators for arrhythmias. Available at www.nice.org.uk/pdf/Defibrillators_A4_ summary.pdf (accessed 1 February 2005).