Wikimedia Commons
Scientists have developed a “photoswitchable” version of the diabetes drug glimepiride that is activated through exposure to blue light, allowing insulin release by pancreatic cells to be optically controlled.
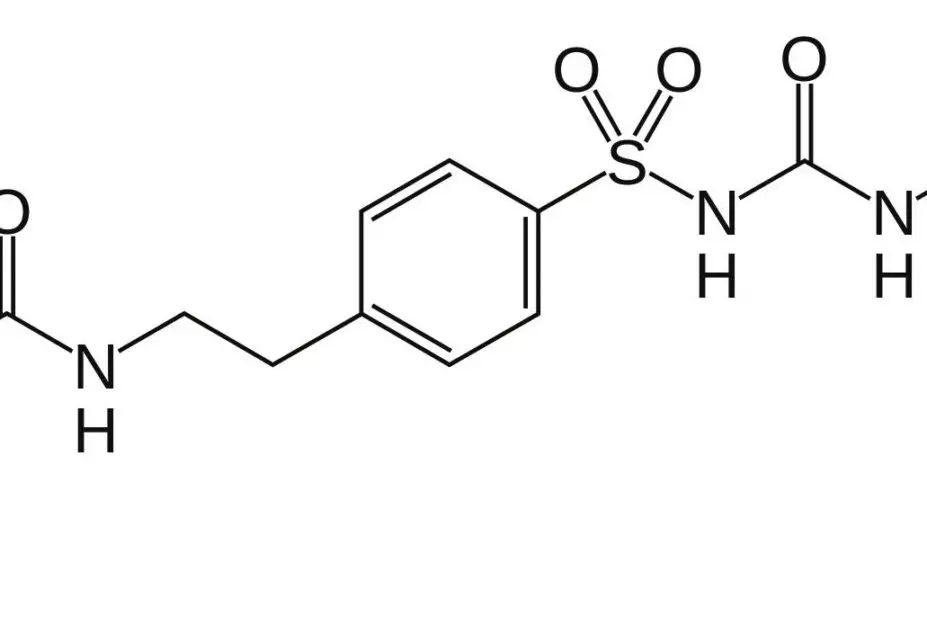
The prototype drug, known as JB253, is an azosulfonylurea compound – a standard sulfonylurea drug (glimepiride) that has been modified to include an azobenzene ring.
In the presence of blue light, this chemical element changes shape, causing the drug to interact with its receptor and exert its biological effect – namely, to act on ion channels in pancreatic islet cells to stimulate insulin release. When JB253 was administered to rodent or human pancreatic islet cells in vitro, application of blue light caused near-instantaneous insulin release, which ceased within milliseconds when the light source was removed.
The drug was developed by an international team based at the Department of Medicine at Imperial College London and Ludwig Maximilian University of Munich. The development of the drug and proof-of-concept study is reported in Nature Communications
[1]
.
The researchers plan to give JB253 to mice; other azosulfonylurea drugs are also in development. Initially, the team will try to activate the drug in vivo using external, high-intensity LEDs (light-emitting diode); if this fails, they plan to implant fibre optics into the animals.
Researcher David Hodson, from Imperial College London, says that while the technology is still at a very early stage, it has the potential to overcome limitations associated with current diabetes therapy. “In principle, this type of therapy may allow better control over blood sugar levels because it can be switched on for a short time when required after a meal,” he says. “It should also reduce complications by targeting drug activity to where it’s needed in the pancreas.”
However, Hodson stresses that photoswitchable diabetes drugs are at least 5–10 years away from being available to patients. Many questions remain unanswered, including issues of safety, pharmacodynamics and how to target the light source to the pancreas. “Nonetheless, we believe that our new drug demonstrates that photopharmacology may one day be useful for the treatment of metabolic diseases such as type 2 diabetes,” he says.
Photoswitchable drugs could be valuable in other areas of medicine. A molecule known as AAQ is about to enter clinical trials as a treatment for retinitis pigmentosa. AAQ works by causing normally “blind” cells in the retina to become sensitive to light, thereby potentially taking the place of dysfunctional rods and cones.
A light-sensitive antibiotic is also in development, which is inactive until it is exposed to ultraviolet light. Such a drug could circumvent the issue of drug resistance caused by existing antibiotics that are excreted unmetabolised and therefore collect in the environment.