BSIP SA / Alamy
The European Medicines Agency (EMA) has recommended the approval of tolvaptan (Otsuka Pharmaceutical Europe’s Jinarc) for the treatment of a rare inherited disease, autosomal dominant polycystic kidney disease (ADPKD), which affects around 205,000 people in the European Union.
The EMA’s Committee for Medicinal Products for Human Use (CHMP) gave the treatment a positive opinion on 26 February 2015, recommending it to be granted a marketing authorisation by the European Commission.
Tolvaptan is indicated to slow down the progression of cyst development and renal insufficiency in adult ADPKD patients with chronic renal disease stages 1 to 3 – that is, normal to moderately-reduced kidney function – at the initiation of treatment, and who have evidence of rapidly progressing disease.
Currently there is no treatment for ADPKD on the EU market, and patient care is limited to managing the symptoms and complications.
Source: PKD International
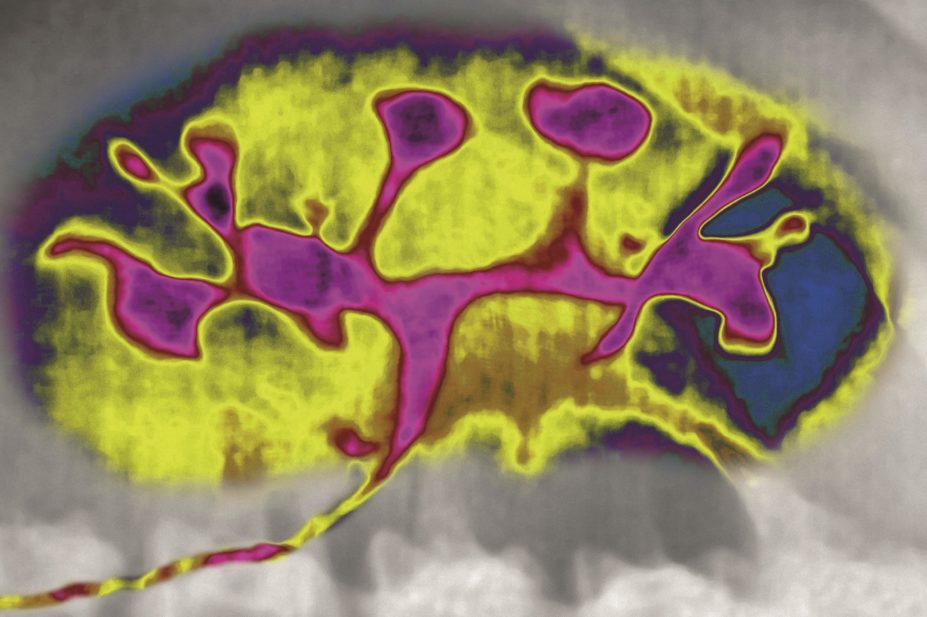
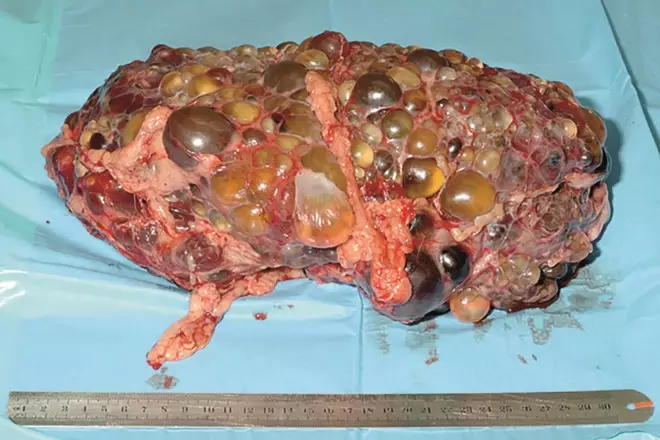
In autosomal dominant polycystic kidney disease (pictured), fluid-filled cysts form because kidney cells do not respond normally to vasopressin, a hormone that regulates the level of water and sodium in the body
In ADPKD, fluid-filled cysts form because, it is thought, kidney cells do not respond normally to vasopressin, a hormone that regulates the level of water and sodium in the body. Tolvaptan is a vasopressin-2-receptor antagonist: it acts by blocking the receptors in the kidneys to which vasopressin attaches, thereby slowing cyst formation.
In a three-year 1,445-patient clinical trial, participants taking tolvaptan showed a 2.8% annual increase in total kidney volume – an indicator of cyst formation and disease progression – compared with 5.5% in the placebo group. Events on worsening kidney function were 61.4% less likely for tolvaptan compared with placebo, the EMA notes.
The EMA has requested some safeguards for patients taking the product. It has recommended hepatic monitoring as part of a registry, since study data showed a significant increase in liver adverse events (2.3% compared with 1% in the placebo group).
Tolvaptan was first approved for ADPKD in Japan in 2015 and was approved in Canada in February 2015, according to its manufacturer. “This recommendation brings us one step closer to providing to patients in Europe the first-in-the-world, disease-modifying treatment for ADPKD, a progressive and chronic genetic disease,” says Ole Vahlgren, chief executive and president of Otsuka Europe.
Tolvaptan is also approved (Otsuka’s Samsca) in the EU for patients with hyponatraemia secondary to syndrome of inappropriate antidiuretic hormone secretion, an indication that got the green light in 2009.
You may also be interested in
Developments in the management of anaemia and other complications in adults with chronic kidney disease

Pharmacy regulator considers giving up legal authority to conduct covert investigations of pharmacists
