
Plume Creative
Our modern lives are longer, but the later years can be fraught. The risk of developing chronic diseases, such as cardiovascular disease, cancer or arthritis, increases steadily with age, which can leave a person frail and trapped in a fog of medical care. And if one disease can be managed, the reality is that others will soon follow.
These chronic diseases threaten healthcare systems and society alike. There are 15 million people with at least one chronic disease in England and 117 million in the United States — nearly half the nation’s adults. They account for 60% of deaths worldwide and the number of those afflicted continues to grow. Managing these long-term conditions soaks up money, with 70% of healthcare spending in England going towards it. This figure leaps to 86% in the United States.
The burden is forecast to grow as populations grow older, spurring a call for an overhaul of how these diseases are managed. In the meantime, families struggle to care for older relatives who live on in ill health, unable to look after themselves.
“All the economies of the world are under stress because of the ageing of the population and rise of these chronic diseases,” says Felipe Sierra, director of the division of ageing biology at the National Institute of Aging (NIA) in Bethesda, Maryland.
Felipe Sierra, pictured, director of the division of ageing biology at the National Institute of Aging (NIA) in Bethesda, Maryland
Although lifestyle factors such as diet and exercise are known to help stave off these illnesses, other approaches are needed. “It’s a matter of economics that we have to do something because people won’t do what they’re supposed to,” Sierra adds. “It’s not just good for them, but good for society.”
It’s a matter of economics that we have to do something because people won’t do what they’re supposed to
Finding cures for individual diseases could help, but Sierra and others have set their sights on ageing, a shared risk factor. To them, slowing the rate of biological ageing may be the best, if somewhat audacious, bet for warding off these debilitating diseases. The goal is to increase ‘healthspan’ — the number of years a person is in good health — rather than simply adding years to life.
“Pharmaceuticals that have an actual effect on the ageing process, and so slow the rate of incidence and progression of a wide range of diseases, ultimately will be far more effective in obtaining healthy active life than ‘science-fictiony’ ideas, like a complete cure for cancer or heart attacks,” says Richard Miller, a researcher on ageing at the University of Michigan.
Armed with the knowledge that basic ageing processes are conserved across species such as yeast, worms, flies and mice, researchers have started to think about translating these insights to humans. Several compounds, including rapamycin, extend lifespan in mice, but human trials await shifts in regulatory practices that currently shun treatment of ageing as a medical condition. An opportunity may come through clinical trials of diabetes drug metformin, which could reveal delays in the onset of age-related diseases. Yet most research has a disease-specific bent that overlooks ageing as a risk factor that can be mitigated.

Matt Kaeberlein from the University of Washington, Seattle
“It hasn’t yet pervaded the common consciousness that ageing is something malleable,” says Matt Kaeberlein from the University of Washington in Seattle. “But the basic biology of ageing has matured enough that translational approaches are, if not here, very close to where we can intervene in the ageing process in a meaningful way.
The basic biology of ageing has matured enough that translational approaches are very close to where we can intervene in the ageing process in a meaningful way
“To me, that is a more efficient approach to improving quality of life than individually trying to cure diseases,” he adds.
Of scarcity and survival
As cells age, their molecular housekeeping wanes: unwanted proteins litter the cell and damaged DNA goes unrepaired. Eventually, aged cells enter a limbo-like state of senescence, in which they stop dividing, but do not die. The stem cells tasked with replacing ageing cells also decline, compromising the body’s ability to renew itself.
Curiously, controls for these ageing processes within the cell have been revealed by going without: decreasing food calories by 30% extends lifespan in diverse species, including yeast, worms, flies and rodents, which can live up to 50% longer[1]
. Caloric restriction also works for monkeys and possibly humans, as suggested by the long-lived but somewhat underfed people of Okinawa, Japan, an idea now being tested in a US study[2]
.
Most people will not adopt such dietary austerity, but the effect has allowed researchers to grasp the biological processes that underlie ageing. With food deprivation, cells seem to go into survival mode, putting off plans for growth and renewal until food is less scarce. Insulin-related pathways and hormones, such as insulin growth factor 1 (IGF1), are downregulated; the target-of-rapamycin (TOR) pathway, which normally promotes growth and suppresses clearance of old proteins from the cell, is reined in; and stress-related transcription begins, making cells more resistant to oxidative stress, heavy metal exposure, heat or DNA damage.
Drugs that work upon these pathways could slow ageing and its associated ailments in people when they need it most.
“It’s hard enough to tell 20-year-olds to wear seat belts, let alone what they should do to feel healthy when they’re 85,” says James Kirkland, a researcher on ageing at the Mayo Clinic in Rochester, Minnesota. “So it’d be great if there was a pharmacologic agent that works for people when they are already beginning to feel that things are going wrong.”
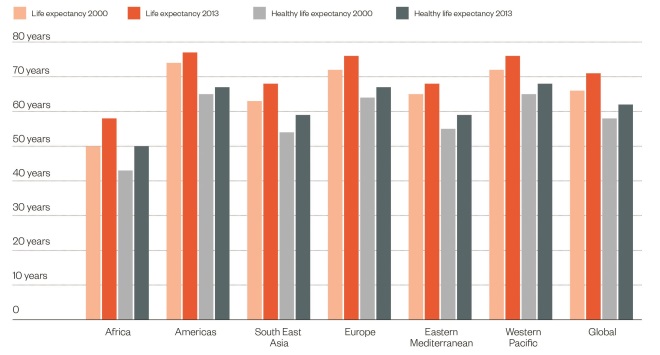
Health lags behind
Source: World Health Organization
Although life expectancy at birth has increased over the past decade, there remains a gap between life expectancy and healthy life expectancy, which lags behind by nine years
Methuselah’s medicine cabinet
Testing compounds for effects on lifespan may seem straightforward enough, but human studies would take hundreds of years to complete. Short-lived species such as mice can give more immediate answers, but differences in experimental designs and protocols hinder interpretability. To standardise longevity studies, the NIA organised the Intervention Testing Program (ITP), which tests five compounds yearly in genetically heterogeneous mice, both males and females, in three different laboratories.
“The literature is filled with reports from just one site that were never confirmed again,” says Miller, who runs one of the sites at the University of Michigan.
So far, the ITP has reported several compounds that extend lifespan. These include rapamycin, an inhibitor of the TOR pathway; acarbose, a diabetes drug that blunts blood glucose surges after eating; and 17-alpha-estradiol, a non-feminising estrogen that works for male, but not female, mice. The ITP has not found evidence for the more fashionable green tea extracts or resveratrol, the hyped ingredient in red wine. Although resveratrol has been shown to protect mice from an unhealthy diet, it hasn’t so far extended lifespan in healthy mice.
Rapamycin boasts the most striking effects, lengthening mouse lifespan by up to 25%[3]
. It works even when given later in life, at the mouse equivalent of a 60-year-old person[4]
, and it delays age-related declines in cognition, cardiac function and the immune system[5]
. It can be argued that its pan-organ effects show that rapamycin is indeed slowing ageing, as opposed to merely inhibiting disease.

Richard Miller, an researcher in ageing at the University of Michigan, Ann Arbor
“The general picture is that the reason [the mice] live longer is because they stay healthy,” Miller says. “No drug we’ve tested creates a Dorian Gray situation… where you’d be trapped in a body of a 100-year-old decrepit, sad, sick person for decades.”
No drug we’ve tested creates a Dorian Gray situation… where you’d be trapped in a body of a 100-year-old decrepit, sad, sick person for decades
In humans, rapamycin is used to help prevent organ rejection in transplant patients, raising fears that it may dangerously suppress the immune system, which declines with age. A 2014 study conducted by Novartis, however, suggested otherwise: a rapamycin analogue called everolimus improved the immune response to an influenza vaccine in older people, and mice treated with everolimus gained more protection from the vaccine against the flu[6]
.
Intermittent rapamycin treatment later in life may also be sufficient, Kaeberlein says. He is conducting a ten-week trial of rapamycin in domestic dogs to look for changes in health and lifespan.
“It’s really exciting because the shorter you can get the window of treatment down, or maybe transient recurring treatments, the less likelihood of significant side effects,” he says.
More to metformin
Regulatory challenges mean that a drug with more modest effects than rapamycin may be the first to be tested in humans. A trial of metformin, which has a slight, if any, effect on mouse lifespan[7]
, won a blessing from the US’s Food and Drug Administration (FDA) in 2015.
Banned by law from evaluating drugs to treat ageing, the FDA has a drug approval process organised around finding treatments for a target disease. The new trial, TAME (‘Targeting aging with metformin’), is subtly different in that it asks whether metformin will delay the development of multiple age-related chronic diseases in people who already have one. If successful, it might convince regulators to consider approving a single drug for multiple diseases.

James Kirkland, pictured, a researcher in ageing at the Mayo Clinic in Rochester, Minnesota
“Anybody would be reluctant to say we are treating ageing, that’s way too much of a stretch,” says Kirkland, who is involved with the TAME trial. “But what is reasonable and what is needed is a way of treating multi-morbidity, that is, the many people who have three or four or five conditions simultaneously, many of whom are elderly.”
What is needed is a way of treating multi-morbidity
The choice of metformin is strategic. It is cheap and safe, with a 60-year track record in humans, and it seems to work on some of the same processes implicated in ageing. A recent observational study bolstered its case by finding that people with type 2 diabetes taking metformin live around 15% longer than non-diabetics not taking metformin[8]
.
While the TAME trial looks for funding, another metformin trial underway in the UK may pick up effects on lifespan. Since metformin given to people with diabetes reduces incidence of heart disease, the trial, GLINT (‘Glucose lowering in non-diabetic hyperglycaemia trial’), asks whether giving metformin earlier to people with high blood sugar but who have not yet developed diabetes, can do the same. The trial plans to enroll nearly 13,000 people, and monitor the development of heart disease and other conditions over five to seven years.
In sickness and in health
As ageing is widely considered a natural part of life, there is a low tolerance for side effects of age-targeting compounds. But given the risks of doing nothing, that caution may eventually give way to tests of newer compounds. Kirkland and colleagues have been developing ‘senolytics’ that target senescent cells[9]
, which are thought to drive some ageing processes[10]
. Parabiotic experiments show that the blood of young mice reinvigorates old mice, and researchers are now seeking the key ingredients in young blood, such as the protein GDF11. Others are developing activators of sirtuin receptors, which spur cellular clean-up, called autophagy, thought to promote longevity.
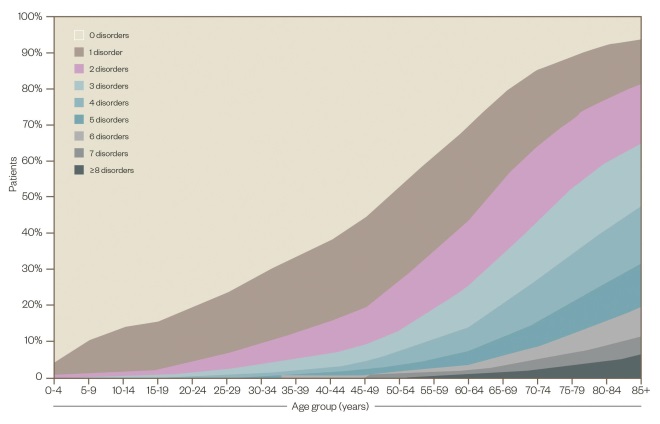
The burden of chronic disease
Source: Barnett K, Mercer SW, Norbury M, et al. The Lancet 2012;380:37-43.
Chronic diseases threaten healthcare systems and society alike. By the age of 50 years, half of the population has at least one chronic condition, and by 65 years, most have multiple conditions
Ageing researchers also want their disease-focused colleagues to consider age in their experiments. Most drugs are tested in young animals, whose physiology differs from older animals. “If you have a treatment that is tested only in young animals, your chances of being properly translated in old humans is low,” says Sierra, noting a cancer immunotherapy that had showed promise in young animals but which was found to be lethal in older animals only after a trial in humans had been halted[11]
.
Sierra started a Geroscience Interest Group at the National Institutes for Health to encourage disease-focused agencies to include experiments in older animals. Not only will this improve translational efforts, but it could reveal details of how physiology changes with age — something that would grow an understanding of what it means to be healthy. “We need to focus more on health, and less on disease,” he says.
References
[1] Weindruch R, Walford RL, Fligiel S et al. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. Journal of Nutrition 1986;116:641–654. PMID: 3958810
[2] Ravussin E, Redman LM, Rochon J et al. A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. J Gerontol A Biol Sci Med Sci 2015;70:1097–1104. doi: 10.1093/gerona/glv057
[3] Miller RA, Harrison DE, Astle CM et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13:468–477. doi: 10.1111/acel.12194.
[4] Harrison DE, Strong R, Sharp ZD et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009;460:392–395. doi: 10.1038/nature08221
[5] Ehninger D, Neff F & Xie K. Longevity, aging and rapamycin. Cell Mol Life Sci 2014;71:4325–4346. doi: 10.1007/s00018-014-1677-1
[6] Mannick JB, Del Giudice G, Lattanzi M et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med 2014;6:268ra179. doi: 10.1126/scitranslmed.3009892
[7] Martin-Montalvo A, Mercken EM, Mitchell SJ et al. Metformin improves healthspan and lifespan in mice. Nat Commun 2013;4:2192. doi: 10.1038/ncomms3192
[8] Bannister CA, Holden SE, Jenkins-Jones S et al. Can people with type 2 diabetes live longer than those without? A comparison of mortality in people initiated with metformin or sulphonylurea monotherapy and matched, non-diabetic controls. Diabetes Obes Metab. 2014;16:1165–1173. doi: 10.1111/dom.12354
[9] Zhu Y, Tchkonia T, Pirtskhalava T et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 2015;14:644–658. doi: 10.1111/acel.12344
[10] Baker DJ, Childs BG, Durik M et al. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature 2016. doi: 10.1038/nature16932
[11] Bouchlaka MN, Sckisel GD, Chen M et al. Aging predisposes to acute inflammatory induced pathology after tumor immunotherapy. J Exp Med 2013;210:2223–2237. doi: 10.1084/jem.20131219
You may also be interested in

Pharmacists have vital role in reducing pressures on health system, says leading pharmacist

Pharmacy regulator considers giving up legal authority to conduct covert investigations of pharmacists
