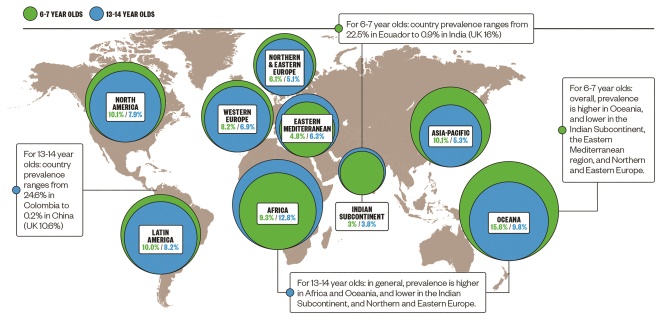
Epidemiology of atopic dermatitis
Source: Journal of Clinical Immunology; Allergologia et Immunopathologia
There is a wide variation in the global prevalence of atopic dermatitis, but it is common in both developed and developing countries.
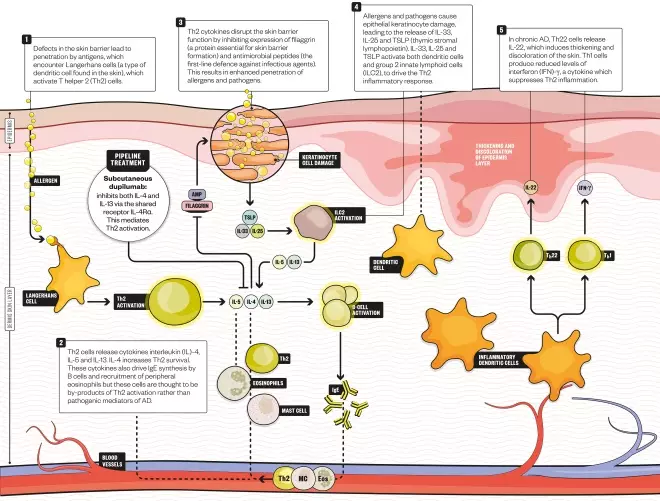
How dupilumab works
Source: Expert Opinion on Biological Therapy; Nature Reviews Drug Discovery; Immunotherapy
The arrival of the first monoclonal antibody for atopic dermatitis, dupilumab, marketed as Dupixent, is anticipated as a revolution for patients with the most severe form of the disease. Dupilumab targets the Th2 pathway that drives atopic dermatitis
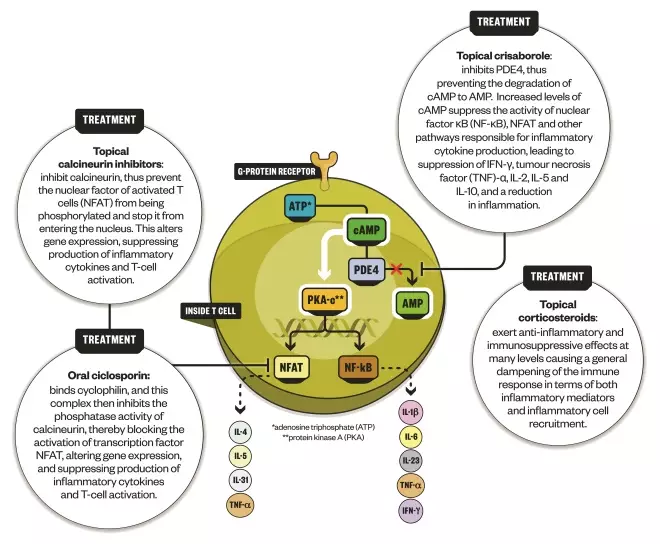
How crisaborole works
Source: Expert Opinion on Biological Therapy; Nature Reviews Drug Discovery; Immunotherapy
For mild disease, topical ointment crisaborole secured US Food and Drug Administration approval in December 2016. Crisaborole works by inhibiting the enzyme phosphodiesterase 4 (PDE4), which has long been known to have a role in atopic dermatitis
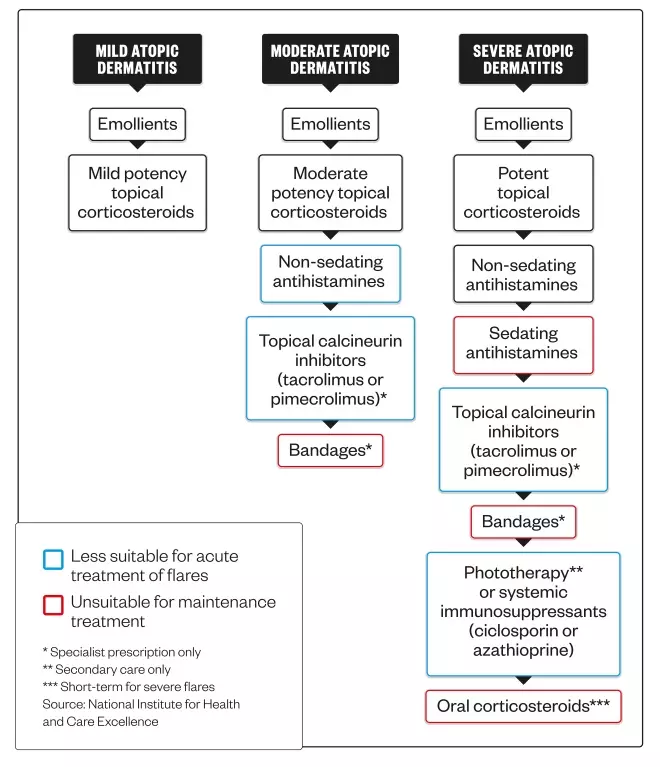
Current stepped treatment options for atopic dermatitis
Treatment for atopic dermatitis can be stepped up or down depending on disease severity. Acute flares will often require a temporary increase in the intensity of treatment
References
Editorial advisers: Tess McPherson, a consultant dermatologist at the Churchill Hospital in Oxford; Amy Paller, director of Northwestern University’s Skin Disease Research Center in Chicago, Illinois.
Graphics: alisdairmacdonald.co.uk