Download a PDF of the full information graphic.
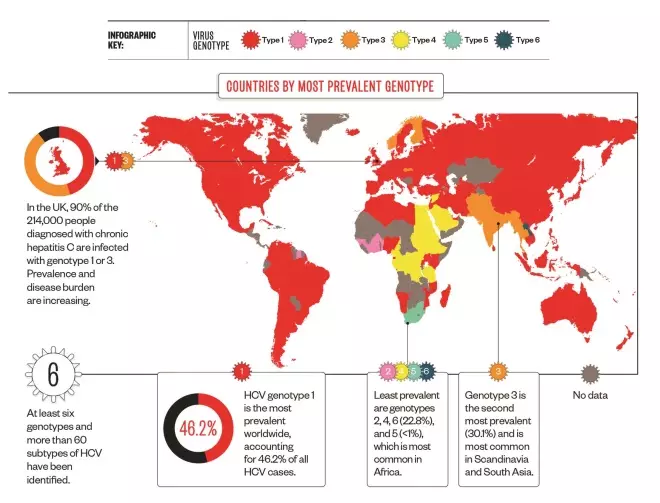
Travelling the world
The hepatitis C virus (HCV) is bloodborne and in developed countries is most commonly transmitted by sharing needles or other drug-injecting equipment. Acute infection is usually asymptomatic, but around 75–85% of people go on to develop chronic infection and, if left untreated or if treatment fails, 5–20% develop cirrhosis within 20–30 years and 1–5% die from cirrhosis or liver cancer.
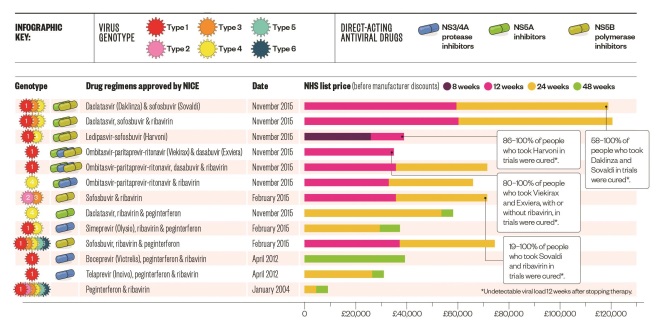
Balancing costs with benefits
For more than a decade, standard therapy was pegylated interferon and ribavirin, which had severe side effects and was only effective in around 50% of genotype 1 and 75% of genotype 2/3 patients. Boceprevir and telaprevir, which target HCV specific proteins, improved cure rates to about 70% in genotype 1 patients, but worsened side effects. Simpler and shorter interferon-free regimens have now been approved by the National Institute for Health and Clinical Excellence (NICE), with higher cure rates (most >90%) and fewer side effects. Regimen choice and length depends on several factors, including genotype, presence of cirrhosis, treatment history, potential drug interactions, HIV or hepatitis B co-infection, NICE guidance, and commissioning arrangements.
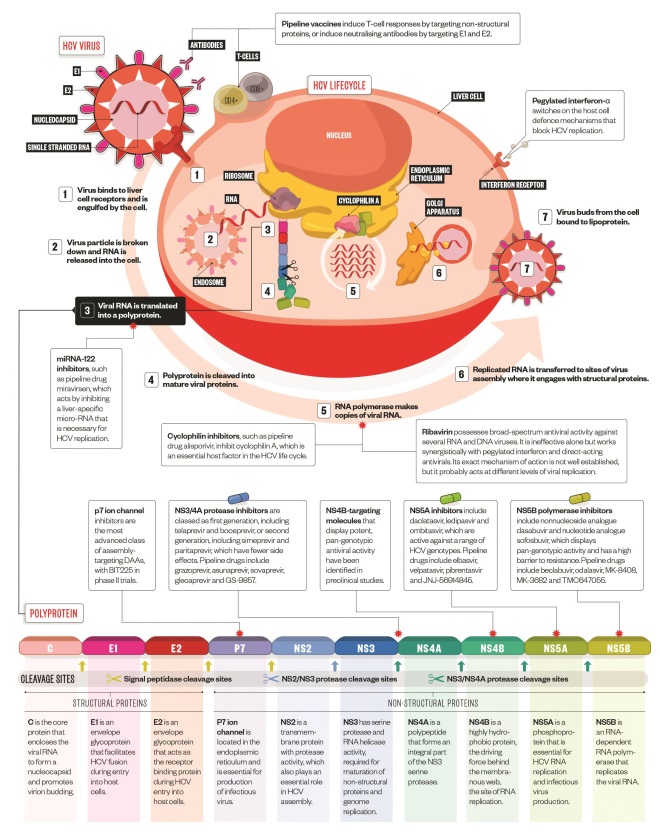
Viral replication and drug targets
HCV is a single stranded RNA flavivirus, which constantly mutates, enabling it to escape attack by the immune system. The HCV genome is translated to produce a polyprotein, which is further processed by host and viral proteases to produce three structural and seven non-structural proteins. A greater understanding of the HCV genome and proteins has enabled the development of direct-acting antivirals.
References
Sources: Travelling the world: Hepatology; Balancing costs with benefits: NICE, BNF, electronic Medicines Compendium, MIMS; Viral replication and drug targets: BioMed Research International, Viruses, Advances in Hepatology, aidsinfonet, Hepatitis C viruses: genomes and molecular biology.
Editorial advisers: John McLauchlan, Centre for Virus Research, University of Glasgow; Sarah Cripps, Oxford University Hospitals NHS Trust; Sarah Knightly, King’s College Hospital NHS Foundation Trust.
You may also be interested in

How we treated more than 100 hepatitis C patients at pharmacist-led clinics in North Wales
Community pharmacies have an essential role in sustaining the elimination of hepatitis C in England