Wikimedia Commons / HeNe
Mepolizumab (Nucala; GSK), the first biologic asthma treatment to target specific white blood cells called eosinophils, has been recommended in draft guidance by the National Institute for Health and Care Excellence (NICE) for adults with severe asthma, after GSK reduced the price for its injectable product.
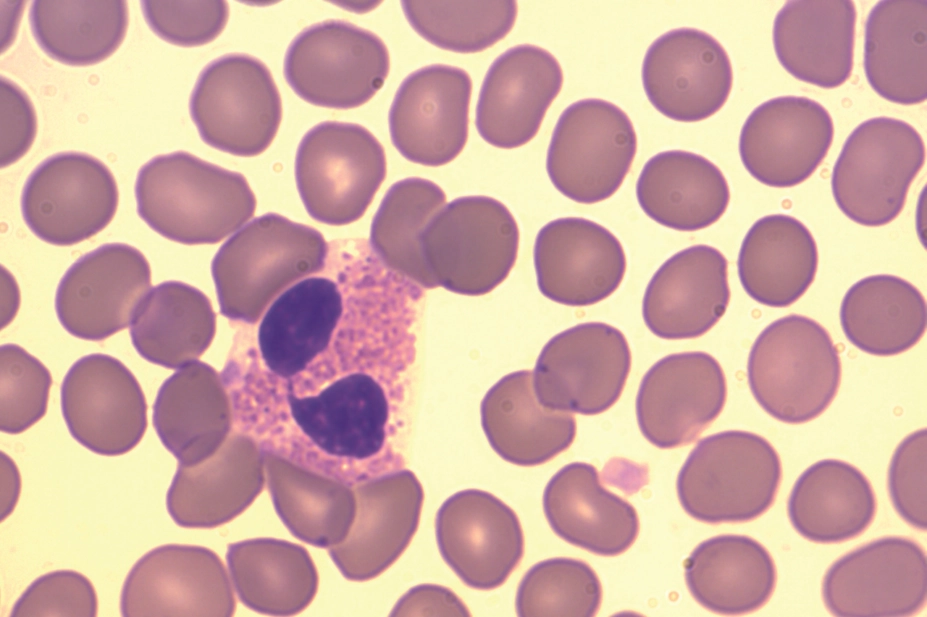
Increased levels of eosinophils are responsible for symptoms in some asthma patients. They produce a signal molecule called IL-5 that helps them survive while attracting more of them to the same area. Mepolizumab stops IL-5 from working and helps reduce the number of eosinophils in the airways.
NICE, the health technology assessment body that provides guidance on which drugs should be used on the NHS in England, has recommended mepolizumab as an add-on treatment for treating severe refractory eosinophilic asthma in adults if they have had a blood eosinophil count of 300/µl or more in the past 12 months despite taking their regular medicines.
The drug is only recommended for eosinophilic asthma if the person has had four or more attacks in the previous 12 months, or if they are taking maintenance oral corticosteroids. Their treatment should also be reviewed annually and stopped if it is providing no benefit.
Mepolizumab is given by injection every four weeks. Its list cost is £840 per dose, but the NHS has negotiated a lower price.
NICE’s appraisal committee previously did not recommend mepolizumab because GSK’s evidence previously suggested the drug would be used in less severe cases of asthma and would not therefore be cost effective. However, GSK then provided further analyses on its use and offered an additional price reduction.
Carole Longson, director of NICE’s health technology evaluation centre, says: “People with severe asthma have had limited treatment options. Many end up taking oral corticosteroids for prolonged periods which can cause further complications such as diabetes, high blood pressure and mood swings.
“Many of these people will soon have access to an extra treatment option to help them take control of their asthma,” she adds.
In response to the announcement, charity Asthma UK has urged health chiefs to ensure that mepolizumab is made available to those who need it “as soon and as widely as possible”.
Kay Boycott, its chief executive, says: “[The drug] has the potential to transform the lives of many people with one of the most debilitating forms of asthma. Not only should it improve some people’s symptoms and reduce the risk of life-threatening asthma attacks, but we also hope it will reduce their reliance on high doses of corticosteroids which can have unpleasant and harmful side effects in the long term.”
The charity notes that out of the 5.4 million people with asthma in the UK, around 100,000 have severe eosinophilic asthma for whom this treatment is targeted towards.
NICE is also appraising reslizumab (Cinqaero) to treat severe eosinophilic asthma and has asked its manufacturer, Teva, to provide more information on the cost-effectiveness of the drug.