Shutterstock.com
After reading this article, you should be able to:
- Understand the basics of acid-base balance;
- Interpret arterial blood gas results;
- Make treatment decisions, including where drug treatment may be required to correct acid-base imbalance.
Maintaining the pH of blood is essential for normal bodily function; however, several clinical scenarios can result in disruption of the body’s acid-base balance. Monitoring of acid-base balance is done by testing patients’ arterial blood gases (ABGs). The results of ABG testing will often influence the treatment that patients receive.
Monitoring ABGs can be useful to:
- Assess the effectiveness of pulmonary gas exchange;
- Identify the presence of metabolic acidosis and alkalosis;
- Identify critically unwell patients requiring urgent intervention;
- Guide treatment and monitor response[1–5].
A basic understanding of how to interpret ABG results can therefore be useful for pharmacists to help them to clarify the clinical picture.
The basics of acid-base balance
The optimal physiological pH of extracellular fluid is 7.35–7.45. A pH outside this range can cause protein denaturation and enzyme inactivation[6]. Because pH is a logarithmic scale, a small change in pH reflects a large change in hydrogen ion (H+) concentration[6]. The following equilibrium equation is crucial to understanding acid-base balance:
H2O + CO2 ↔ H2CO3 ↔ HCO3– + H+
This equation shows that carbon dioxide (CO2) in blood dissolves to form carbonic acid (H2CO3), which dissociates to form acidic H+ (which can then combine with physiological bicarbonate to push the equation back to the left). Blood pH depends on the balance of carbon dioxide and bicarbonate (HCO3–). A change in the amount of carbon dioxide will not lead to a change in pH if it is accompanied by a change in the amount of bicarbonate that preserves the balance (and vice versa)[2]. It is the renal and respiratory systems that are responsible for maintaining the pH of the blood.
The following equation can be used to estimate pH:
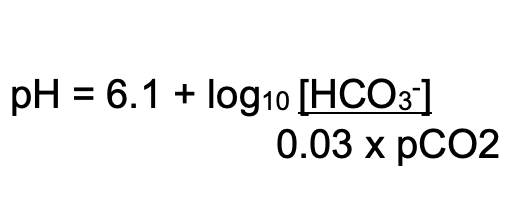
Respiratory mechanisms
Carbon dioxide behaves as an acid in aqueous solution. One way that the body controls the pH of extracellular fluid is by increasing or decreasing the rate and depth of respiration and thereby the amount of carbon dioxide expelled. The partial pressure of carbon dioxide in arterial blood (PaCO2) reflects the amount of carbon dioxide in arterial blood. PaCO2 is determined by alveolar ventilation[2,4]. Chemoreceptors located in the medulla sense pH changes in extracellular fluid and changes in arterial carbon dioxide, altering ventilation to maintain normal pH. In patients with chronically high PaCO2 (chronic hypercapnia); for example, owing to severe chronic obstructive pulmonary disease (COPD), chemoreceptors may become desensitised. The body then relies on PaO2 to detect inadequate ventilation (hypoxic drive)[2,7]. In chronic hypercapnia, supplemental oxygen in emergency settings should be given in a controlled manner with ABG monitoring to avoid respiratory depression[3].
Renal (metabolic) mechanisms
Another way that the body can control pH is through the kidneys, by:
- Excretion of hydrogen ions;
- Renal tubular reabsorption of bicarbonate ions.
The kidneys can adjust the amount of hydrogen and bicarbonate that is excreted in the urine in response to metabolic acid production. However, kidneys also preserve electroneutrality by maintaining stable concentrations of major electrolytes, such as potassium and sodium. This function can sometimes take priority over pH regulation. Normally, sodium (Na+) ions are retained in exchange for potassium (K+) or hydrogen ions. When potassium ions are in short supply, more hydrogen ions are excreted, which can interfere with pH regulation. Another kidney function which can affect acid-base balance is the maintenance of negatively charged chloride (Cl–) and HCO3– ions to balance positively charged ions. When chloride ions are in low supply, more bicarbonate is retained and vice versa[2].
Compensation
When acidosis or alkalosis occurs (either through respiratory or renal mechanisms; see Table 1), the opposite system will attempt to rectify this imbalance; this is termed ‘compensation’. For example, if the kidneys fail to excrete metabolic acids, ventilation is adjusted to eliminate more carbon dioxide[2].
It is important to note that compensatory changes in respiration can occur over minutes to hours, whereas metabolic processes take hours or days to respond[2,8].
Buffers
The body has three main buffers that minimise any changes in pH that occur when acids or bases are added: haemoglobin, bicarbonate and proteins. Haemoglobin is six times more powerful as a buffer than proteins[6]. Bicarbonate is the most important buffer in the blood and is the dominant buffer in the interstitial fluid. The intracellular fluid uses proteins and phosphate to buffer pH[8]. At an intracellular level, buffering occurs instantly but the effect is small.
Arterial blood gas sampling
Commonly reported parameters of ABG results (see Table 2 for the normal reference ranges[2]) include:
- pH — to determine whether a patient’s blood pH is within physiological range;
- PaCO2 and PaO2 — the partial pressures of CO2 and oxygen in arterial blood, respectively;
- Bicarbonate — indicates how much bicarbonate is in the blood and is therefore available as a buffer;
- BE (base excess or deficit) — a measure of the excess or deficiency of base in the blood; by definition, it is the amount of base (in mmol) that would correct one litre of blood to a normal pH of 7.4. If an excess, this is the amount of base needed to be removed for a normal pH, or, if a deficit, the amount required to be added.
- Lactate — the product of anaerobic glycolysis. A rise in lactate indicates poor oxygenation and perfusion of tissues.
Other parameters commonly found on ABG reports are haemoglobin, glucose and electrolytes (sodium, potassium, chloride and ionised calcium).
Assess pulmonary gas exchange
In type one respiratory impairment there is defective oxygenation despite adequate ventilation, characterised by a low PaO2 with a low or normal PaCO2. In patients receiving supplemental oxygen, the PaO2 may be within normal range but inappropriately low for the fraction of inhaled oxygen (FiO2). Common causes of type one respiratory failure include: pneumonia; COPD; acute respiratory distress syndrome; pulmonary embolism; pneumothorax; acute asthma; and pulmonary oedema[2,4].
In type two respiratory impairment there is inadequate ventilation (pumping air in and out of the lungs), characterised by a high PaCO2 and low PaO2. In patients receiving supplemental oxygen, the PaO2 may be within normal range. Common causes of type two respiratory impairment include: COPD; opioid or benzodiazepine toxicity; obstructive sleep apnoea; flail chest injury; neuromuscular disorders; and exhaustion following type one respiratory impairment[2,4].
Hyperventilation is characterised by a low PaCO2. Accompanying low HCO3– indicates hyperventilation secondary to metabolic acidosis or, rarely, chronic hyperventilation with metabolic compensation[2].
Interpreting the results
ABGs can be interpreted using a stepped approach.
Step 1 — check the pH
The pH should be assessed first. A pH of less than 7.35 indicates acidaemia — acid in the blood, and a pH greater than 7.45 indicates alkalaemia — alkali in the blood.
Step 2 — check the HCO3– and PaCO2
Having determined if the patient is acidotic or alkalotic, check the bicarbonate and the PaCO2 (refer to Table 3) to classify the results as follows:
- Metabolic acidosis: patients who are acidotic and have a low bicarbonate and increased base deficit. There is usually a compensatory increase in alveolar ventilation to lower PaCO2. A high chloride indicates hyperchloremic acidosis;
- Respiratory acidosis: patients who are acidotic with a high PaCO2. Because metabolic compensatory response takes longer to develop, acute respiratory acidosis is almost always uncompensated;
- Metabolic alkalosis: patients who are alkalotic with a high bicarbonate and an increase in base excess. Respiratory compensation (increase in PaCO2) occurs but is limited by the need to avoid hypoxaemia;
- Respiratory alkalosis: patients who are alkalotic with a low PaCO2. Metabolic compensatory response takes longer to develop[2].
It is possible for patients to have a mixed respiratory and metabolic alkalosis or acidosis. This occurs when primary respiratory and primary metabolic disturbances exist simultaneously. If the two processes oppose each other, pH derangement will be minimised (see Step 3). However, two processes that cause pH to move in the same direction may lead to profound acidaemia or alkalaemia[2].
Step 3 — Check for compensation
Check to see if the patient is compensating for their acid-base imbalance. Patients may partially or fully compensate for an acid-base imbalance by the “opposite” mechanism; for example, metabolic acidosis will be compensated for with respiratory alkalosis. This may create some apparently normal results among some deranged ones. Compensation is greater in chronic disorders. Overcompensation does not occur[2,4].
When interpreting acid-base status, it is important to always take the clinical context into account. For example, if presented with ABG results showing a normal pH, low PaCO2 and low bicarbonate in a diabetic patient with high levels of ketones in the urine, the most likely primary disorder is metabolic acidosis (diabetic ketoacidosis), rather than respiratory alkalosis (see Table 3)[2,5].
Step 4 — Calculate the anion gap
For a patient with metabolic acidosis, it can be useful to calculate the anion gap as this can give some indication of the underlying cause of the acid-base imbalance.
The anion gap is the difference between the measured positively charged cations Na+ and K+ and the negatively charged anions Cl– and HCO3–[6]. The following equation can be used to estimate the anion gap:
([Na+] + [K+]) – ([Cl–] + [HCO3–])
Some laboratories do not include potassium in the calculation, which affects the accepted range. An increased anion gap indicates excess acid from the anions that are unmeasured (e.g. ketones or lactate)[4]. These may be endogenous (e.g. ketones or lactate) or exogenous (e.g. ethylene glycol, aspirin overdose). It is also worth noting that a drop in a patient’s albumin lowers the anion gap. A deranged phosphate level can also affect the anion gap, but to a lesser extent[5,9].
Treatment
If possible, the underlying cause of the acid-base derangement should be treated because, without doing so, the problem can recur. Depending on the cause, this may include: treating infection; switching intravenous fluid (e.g. to reduce chloride load); managing diarrhoea or high stoma output; reversal of respiratory depressant drugs; or managing drug overdose. In some instances, it may not be possible to treat the underlying cause and drug treatment may be required to correct the acid-base imbalance.
Drugs used to treat metabolic acidosis include sodium bicarbonate and trometamol (THAM).
Sodium bicarbonate is most commonly used in patients with chronic kidney disease, with National Institute for Health and Care Excellence guidance recommending oral supplementation in patients with an estimated glomerular filtration rate <30mL/minute and a bicarbonate of <20mmol/L[10]. It increases the patient’s bicarbonate concentration as well as reduces the potassium concentration. Intravenous infusions of sodium bicarbonate may be given for severe metabolic acidosis, particularly associated with hyperkalaemia, including peri-arrest situations. The dose is calculated according to base deficit and care should be taken not to over-correct the imbalance, causing an alkalosis. The following calculation is used to calculate the required extracellular buffer:
Body weight (kg) x 1/5 x base deficit = sodium bicarbonate (mmol) dose
In practice, twice the dose is required to facilitate intracellular buffer activity. As over-correction may have adverse effects on potassium and calcium concentration, it is safer to achieve a pH of 7.2 and reassess whether there is clinical need for more[11].
Intravenous solutions of sodium bicarbonate come in concentrations ranging from a 1.26% iso-osmolar preparation to the hyperosmolar 8.4% preparation. Hyperosmolar preparations are very irritant to veins and should usually be administered through a central line, with the exception of a peri-arrest situation. Sodium bicarbonate is also used as a buffer in dialysate and replacement fluid for renal replacement therapy in patients with acute kidney failure and liver failure and/or lactic acidaemia/or circulatory shock[12].
THAM, a proton acceptor, is an unlicensed medicine used in patients unable to tolerate the high sodium load associated with intravenous sodium bicarbonate. THAM acts as a buffer in metabolic acidosis. It is rarely used in current clinical practice[5,13]. Patients with respiratory alkalosis may be given acetazolamide, a carbonic anhydrase inhibitor, which promotes the excretion of bicarbonate in the urine[11]. This is commonly used in the prevention and treatment of altitude sickness.
Further learning
Test yourself: practice examples
Consider the following patient examples and try to identify which type of disorder is affecting their blood gases. When you have answered all of the questions, click on ‘Finish quiz’ to see your results. For any incorrect answers you will have the option to restart the quiz or access answer guidance from the ‘View questions’ option.
This is an updated version of an article previously published in The Pharmaceutical Journal in March 2011.
- 1Arterial blood gases: a guide to interpretation. BMJ Learning. 2022.https://learning.bmj.com/learning/search-result.html?channelCode=foundationprogramme&channelFamilyConfig=bmj&moduleId=5004327&searchTerm=%E2%80%9Cblood%20gas%E2%80%9D&page=0&utm_source=google&utm_medium=cpc&utm_campaign=usage&utm_content=inst_nhs-wales&gclid=Cj0KCQiAw8OeBhCeARIsAGxWtUy9OlY0yRPo88n7qp2s1qj3Du9t8h_3DPJhf6nzHMV6UT1Rq3DrYHsaAliLEALw_wcB (accessed Feb 2023).
- 2Hennessey I, Japp A. Arterial blood gases made easy. 2nd ed. Elsevier 2015.
- 3O’Driscoll BR, Howard LS, Earis J, et al. British Thoracic Society Guideline for oxygen use in adults in healthcare and emergency settings. BMJ Open Resp Res. 2017;4:e000170. doi:10.1136/bmjresp-2016-000170
- 4Burns GP. Arterial blood gases made easy. Clin Med. 2014;14:66–8. doi:10.7861/clinmedicine.14-1-66
- 5Al-Jaghbeer M, Kellum JA. Acid–base disturbances in intensive care patients: etiology, pathophysiology and treatment. Nephrol. Dial. Transplant. 2014;30:1104–11. doi:10.1093/ndt/gfu289
- 6Baylis C, Till C. Interpretation of arterial blood gases. Surgery 2009;27:470–474.
- 7McCartney A, Phillips D, James M, et al. Ventilatory neural drive in chronically hypercapnic patients with COPD: effects of sleep and nocturnal noninvasive ventilation. Eur Respir Rev. 2022;31:220069. doi:10.1183/16000617.0069-2022
- 8Atherton JC. Acid–base balance: maintenance of plasma pH. Anaesthesia & Intensive Care Medicine. 2009;10:557–61. doi:10.1016/j.mpaic.2009.08.005
- 9Wargo KA, Centor RM. ABCs of ABGs: A Guide to Interpreting Acid-Base Disorders. Hosp Pharm. 2008;43:808–18. doi:10.1310/hpj4310-808
- 10Chronic kidney disease: assessment and management. National Institute for Health and Care Excellence. 2021.https://www.nice.org.uk/guidance/ng203 (accessed Jan 2023).
- 11Waldmann C, Soni N, Rhodes A, et al., editors. Oxford Desk Reference: Critical Care. Oxford: : Oxford University Press 2019.
- 12KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney International Supplements. 2012.https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf (accessed Feb 2023).
- 13Hoste E, Colpaert K, Vanholder R, et al. Sodium bicarbonate versus THAM in ICU patients with mild metabolic acidosis. J Nephrol 2005;18:303–7.https://www.ncbi.nlm.nih.gov/pubmed/16013019