Dr Steven A Rosenberg, MD/PhD
Robert Hershberg, chief scientific officer at New Jersey-based biotechnology company Celgene, recalls the first time he heard about engineered T cells. It was back in 2002 when he was working at a company called Corixa in Seattle, Washington. “There was a discussion about these strange molecules called T bodies and we all thought it was a little bit hare-brained and I remember thinking… it’s going to be a real longshot.”
T cells, a type of lymphocyte, scan our bodies for infection or disease, killing cells that present any tell-tale signs of a virus or malfunction. The field of engineered T cells is based on a simple concept: take a patient’s own T cells, engineer them to target a particular cancer and multiply them in the lab, before infusing them back into the patient.
Hershberg’s scepticism about engineered T cells turned to excitement in 2011 when the University of Pennsylvania released data from one of the first clinical trials using engineered T cells to treat patients with advanced chronic lymphocytic leukaemia who had stopped responding to chemotherapy. In three patients, infusions of engineered T cells cleared over 1kg of tumour cells from each of their bodies. Two patients had complete eradication of their disease, while a third patient had a partial response[1]
. The T cells are able to carry on living in the body, continuing to fight disease.
“You just couldn’t help notice that astonishing clinical response, it almost took people’s breath away,” says Hershberg. News of the results spread. “You’d go to these meetings and have whole rooms of people just staring at the CT scans and you’d hear the stories and it was really just unlike anything that people had ever heard. It was clearly something different,” he says.
You just couldn’t help notice that astonishing clinical response, it almost took people’s breath away
Swiss pharmaceutical company Novartis thought so too and licensed the technology from the University of Pennsylvania in 2012. The University of Pennsylvania wasn’t the only university working on engineered T cells and indeed was not the first. Work by New York-based Memorial Sloan Kettering Cancer Center and the Fred Hutchinson Cancer Research Center in Seattle formed a biotechnology company called Juno Therapeutics, also located in Seattle. In June 2015, Celgene bought a 10% stake in the company for US$1bn, the largest biotechnology licensing deal in history.
These deals mark a stark change in fortunes for a research field that was only of interest to a handful of academics and was extremely slow to progress. It has taken almost 25 years to prove the value of engineered T cells. Now, there are multiple biotechnology companies developing the therapy and dozens of new researchers have joined the field, ramping up competition. With pharmaceutical companies now in the mix, a race is on to see who can bring the technology to patients the quickest. But before this can happen, the technology’s safety must be proven — Juno announced in 2016 that three patients had died during a clinical trial.
The beginning
It was in 1989 that Zelig Eshhar, a scientist at the Weizmann Institute of Science in Tel Aviv, Israel, first had the idea to engineer a patient’s own T cells. Ordinarily, T cells are guided to the site of attack by T cell receptors that sit on their surface. Eshhar introduced segments of DNA-encoding antibodies into the T cells. Antibodies have an exquisite specificity for their targets. Eshhar’s idea was to use this specificity to re-educate T cells to recognise targets that they otherwise wouldn’t.
They are now known as chimeric antigen receptor T (CAR-T) cells. “It’s a bit like the chimera, from the Greek tradition, part one animal part another animal — it’s part T-cell receptor and part antibody,” says Renier Brentjens, director of cellular therapeutics at Memorial Sloan Kettering. The second generation of CAR-Ts are even more complex. They contain additional domains from co-stimulatory molecules — helper proteins that amplify the CAR-T cell response when it meets its target.
It’s a bit like the chimera, from the Greek tradition, part one animal part another animal — it’s part T-cell receptor and part antibody
Brentjens started working on CAR-T cells in 1999 when he joined the laboratory of Michel Sadelain at Memorial Sloan Kettering. At the time, Brentjens was training to be an oncologist and had an interest in haematologic cancers, so called “liquid” cancers, which include leukaemia and lymphomas. He had been recruited to help to develop a CAR-T cell targeting CD19, which is expressed only on the surface of B cells, making it an ideal target for B cell cancers such as leukaemia.

Courtesy of Renier Brentjens
Renier Brentjens, director of cellular therapeutics at Memorial Sloan Kettering Cancer Center, started working on CAR-T cells in 1999
In 2003, the team at Memorial Sloan Kettering was the first to prove that the therapy could work in animals. A paper published in Nature Medicine showed that engineered T cells directed to CD19 could eradicate B cell tumours in a mouse model[2]
. But far from impressing other researchers in the field, Brentjens says that at the time “we couldn’t sell this information, no one was really interested”. Very few groups were working on the technology, maybe six or seven around the world, says Brentjens, and it was difficult to get funding. “I remember we’d write grants and the reviewers would write back and say this doesn’t work or it’s not going to work anyway,” he says.
T cell receptor technology
The National Cancer Institute (NCI), part of the National Institutes of Health based in Bethesda, Maryland, has also been working on Tcell therapies for several decades. “Despite my relative youth, when I started this work there were immunologists who thought what we were doing was pseudoscience,” says Christopher Klebanoff, a staff clinician in the experimental transplantation and immunology branch of the NCI.
Some researchers at the NCI have specialised in a different, but related technique, now known as T cell receptor (TCR) technology. In the 1980s, Steven Rosenberg, head of tumour immunology at the NCI, discovered that T cells extracted from melanoma tumours naturally target mutated tumour proteins, while leaving healthy tissue intact in the laboratory. It is now known that the tumour environment stops these T cells from attacking.
Rosenberg had the idea of extracting the T cells, expanding their numbers in the laboratory and re-infusing up to two hundred billion of them back into the patient along with a T cell stimulator, interleukin 2. “It was wild stuff back then,” says Klebanoff.
Rosenberg treated 20 patients with metastatic melanoma and 55% of these experienced regression of their cancer[3]
. Some are still alive today. This technique is still being used, but has had limited success outside of melanoma and has been somewhat eclipsed by new effective treatments.
In the early 1990s, Rosenberg paired up with Eshhar to work on the development of CAR-T cells. But spurred on by the melanoma trials, researchers at the NCI also developed a way to use the body’s natural anti-cancer T cells in a more sophisticated therapy. Sitting on the surface of T cells are T cell receptors that latch on to fragments of diseased proteins. The researchers learned how to identify the T cell receptor that latches on to the patient’s mutated cancer proteins, clone it and put it into young and fit T cells, creating a more potent and precise therapy.
This ‘T cell receptor’ strategy has advantages and disadvantages. It is mostly successful against tumours that have a high burden of mutations, such as melanoma, but it’s not as easy to find sufficiently effective T cells against other types of cancer. “The optimal target is a [protein that is a] product of the mutation that made the cancer,” says Klebanoff, because it means the T cell will not bind to healthy cells. This makes TCR therapy incredibly personalised. In contrast, the ongoing challenge with CAR-T therapy is to find a target that is shared by many patients but is unique to cancerous cells.

Courtesy of Christopher Klebanoff
Christopher Klebanoff, a staff clinician in the experimental transplantation and immunology branch of the National Cancer Institute, has specialised in identifying T cell receptors that target cancer
The TCR technique was first trialled in humans in 2006. Richard Morgan, then at the NCI and vice-president of a biotechnology company called Bluebird, conducted the first human trial to treat melanoma[4]
. “There was tremendous excitement in the lead up to it, but then also apprehension and frustration when only 2 out of 18 patients had an anti-tumour response — it showed we had a lot of work to do,” says Klebanoff.
There was tremendous excitement in the lead up to it, but then also apprehension and frustration when only 2 out of 18 patients had an anti-tumour response
CAR-T cell trials
At the same time as human trials were starting at NCI, scientists at Memorial Sloan Kettering were opening up their CAR-T-cell therapy to patients. But Brentjens says the trials were hard to get off the ground. It required extensive communication with the US Food and Drug Administration (FDA) to organise and get approval for the trial. It took a while to get patients to enrol because the treatment was so novel and the hospital had to raise the money itself. There were also other obstacles, says Brentjens, such as developing the protocols for treating patients from scratch and scaling up production of the CAR-T cells.
The first trial was in chronic lymphocytic leukaemia (CLL) but, similar to the experience at the NCI, Brentjens says the results were “quite modest, probably because we didn’t properly condition the patients”. Conditioning the patients with chemotherapy removes existing T cells from the body that would compete with the engineered T cells, which helps them to survive for longer. The first four CLL patients in the trial had no objective response and the next four patients, who had conditioning with cyclophosphamide, had a response lasting up to six months[5]
.
By this point, it had been almost 20 years since researchers first worked on CAR-T therapy. “Usually if something works great, it doesn’t take that long to show it works in humans,” says Brentjens. And yet, they persevered. Why? Laughing, he says: “Oh because we were convinced it was going to work.” The concept is so simple, so enticing, he explains. “And you really want to make sure it doesn’t work before you give up on it.”
Usually if something works great, it doesn’t take that long to show it works in humans
For Klebanoff, he says the occasional dramatic response from a patient provided powerful motivation to continue. “It only takes seeing one or two patients who had a complete response to an immunotherapy, despite having previously progressed through every standard therapy, to know that something very powerful and very important was waiting to be discovered with just a little more perseverance.”
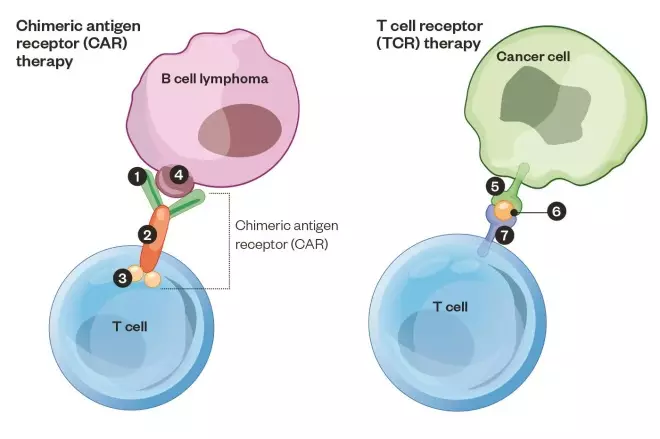
Harnessing T cells to attack cancer
In chimeric antigen receptor (CAR) therapy, a patient’s T cells are harvested from a blood sample and DNA is introduced that encodes the instructions for a CAR, which targets the T cells to B cells. Engineered cells are then infused back into the patient. A CAR developed by Memorial Sloan Kettering against B cell cancer is depicted 1: Antibody fragment: recognises the target protein 2: CD28 fragment: stimulates T cell 3: CD3 fragment: activates T cell, which then kills the B cell 4: CD19 protein: target of the CAR-T. In T cell receptor (TCR) therapy, the patient’s T cells are extracted from their tumour, the cancer-recognising T cell receptor is identified, cloned and the DNA code for this TCR is inserted into the patient’s younger and healthier T cells and then infused back into the patient. 5: Major histocompatibility complex displays cancer protein on cell surface 6: Cancer protein 7: T cell receptor: a clone of the patient’s cancer-recognising T cell receptor targets and activates the T cell so it kills the cancer cell.
Success at last
This moment finally arrived in 2011. It was the University of Pennsylvania that proved the doubters wrong and published the dramatic results that for the first time showed CAR-T cells could eradicate disease in patients who would otherwise have likely died[1]. Similarly to the NCI and Memorial Sloan Kettering groups, the team at the University of Pennsylvania had to rely on money from philanthropists to conduct the first trial. Carl June, professor of immunotherapy at the university, had been working relentlessly on CAR-T cells since the late 1990s and is now regarded as one of the leaders in the field.
Two years later, Memorial Sloan Kettering published the results from a trial of five adults with acute lymphoblastic leukemia (ALL) that had come back after chemotherapy, a disease with a very poor outlook. In 100% of these patients, all signs of the disease had disappeared[6]
. Brentjens says he remembers it was a “delightful” moment the first time he and a colleague checked a patient’s bone marrow sample and couldn’t find any cancer cells. But, he didn’t say, “I can’t believe it works. Because we always believed that it would work.”
The first success at the NCI was in 2014. A trial run by Eric Tran showed that T cells targeting a mutated protein could induce regression of bile duct cancer[7]
. A biotechnology company called Kite Pharma was formed to commercialise the NCI’s TCR and CAR-T work. “It was really Novartis that gave courage to others to pursue this as a way to make money, up until then it was seen as too personalised, so specialised that industry would never want to have anything to do with it,” says Brentjens.
It also means there are now three competing companies. And all of them are working on cell therapies that target CD19 on B cells.
“It’s drawn a lot of attention that there is that much interest from three groups with overlapping technology. So it’s been a bit of a foot race,” says Hershberg at Celgene. Kite now has four products in clinical trials, Juno has eight and Novartis has one CAR-T therapy in multiple phase II trials that has shown a 93% response rate in paediatric patients with ALL .
It’s drawn a lot of attention that there is that much interest from three groups with overlapping technology. So it’s been a bit of a foot race
Overall, about US$3bn has been invested over six years. And in 2015, 180 T-cell therapies were in development. By 2030, the industry is predicted to be worth US$30bn by the business research company Roots Analysis. “I guess there is a certain element of vindication”, says Brentjens. “Being offered posters on the last day at major meetings when no one comes by to look at your work seems funnier now than it did at the time.”
But this success has also brought some controversy to the field, with a lawsuit over the ownership of some of the intellectual property. The original deal between Novartis and the University of Pennsylvania attracted attention but not all of it was positive. St Jude’s Children’s Hospital claimed it had a patent on the DNA designs used by Pennsylvania in its breakthrough 2011 study and in the deal with Novartis. By this point, Juno and St Jude’s had signed a deal to collaborate and so the biotechnology company also waded into the dispute. Eventually, Novartis paid US$12.5m to St Jude’s, the company was also ordered to pay a share of royalties on future products to St Jude’s and Juno.
Competition between the various academic institutions is also fierce. Fred Hutchinson Cancer Research Center, University of Pennsylvania and Memorial Sloan Kettering Cancer Center, among others, have welcomed investment from industry and philanthropy. The University of Pennsylvania now boasts the largest immuno-oncology group working on CAR-T, with 300 researchers (three years ago there were only 40 researchers). And with grant money now easier to secure, new research groups have sprung up. Reflecting on the sudden popularity of the field, Brentjens says: “I guess for the people who started out this race there wants to be some sense that their contribution is acknowledged. I would say that the field is getting very crowded now.”
Safety concerns
After many years, Hershberg says that CAR-T-cell therapy might be just around the corner. The Rocket trial, testing a CD19 CAR-T for ALL, at Juno started in June 2015. “So there should be registration quality data available as early as next year,” he says. However, it has suffered a setback after it was announced on 7 July 2016 that two patients (later revealed to be three) had died. This sent its stock shares down 30%. The deaths were blamed on a new conditioning protocol and after talks with the FDA, the trial resumed using the previous protocol. This is just one of the aspects of CAR-T therapy that still needs to be carefully worked out.

Courtesy of Robert Hershberg
Robert Hershberg, chief scientific officer at Celgene, says that, after many years, CAR-T-cell therapy might be just around the corner
Another aspect is that T-cell therapies are potent. They quickly activate the immune system and can cause cytokine release syndrome, which is like a storm of immune signals. Drugs such as tocilizumab that dampen immune signals can effectively manage this reaction. But their potency means that T-cell therapies must be incredibly targeted. A trial of CAR-T designed to attack the same target as Herceptin, the hugely successful breast cancer drug that targets HER-2, resulted in the onset of respiratory distress within 15 minutes after administration. The HER2 receptor was present at low levels on lung cells and the patient later died[8]
.
This underlines the difficulty of using CAR-T to treat solid cancers. It is hard to find a cancer-specific target that is shared among many patients but is absent from healthy cells. The treatment of B cell cancers has been so successful because they are the only cells that express CD19 and also because a person can live without B cells. The solid tumour environment is immune suppressive and T cells can have trouble penetrating the tumour. Kite is using its TCR strategy for solid tumours but other groups are working on a way to use CAR-T. The University of Pennsylvania has just published a proof-of-concept study in mice that targets glycoproteins specific to tumours.
At Memorial Sloan Kettering, the researchers have also opened up a trial for ovarian cancer. Commenting on the solid tumour work, Brentjens says there are many aspects that still need to be worked out. But he is used to taking on these challenges. “We’re approaching solid tumours with the same conviction we approached liquid tumours; that it should work. So now we’ve just got to make it work,” he says.
Reflecting on the success of the field, Brentjens says it’s “incredibly humbling” to see patients who are alive now because of a therapy he helped develop in the early stages. But he adds: “If you happen to be one of the lucky ones who finds, or picks, or gets assigned to a project where you do actually make a difference, you shouldn’t be arrogant about it, but thank your lucky stars that this project fell to you.”
References
[1] Kalos, M, Levine B, Porter D et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Science Translational Medicine 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842
[2] Brentjens R, LaTouche JB, Santos E et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nature Medicine 2003;9:279–286. doi: 10.1038/nm827
[3] Rosenberg S, Packard B, Aebersold P et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma: a preliminary report. New England Journal of Medicine 1988;22:1676–1680. doi: 10.1056/NEJM198812223192527
[4] Morgan R, Dudley M, Wunderlich J et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006;314:126–129. doi: 10.1126/science.1129003
[5] Brentjens, R, Riviere I, Park J et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540
[6] Brentjens R, Davila M, Riviere I et al. CD19-targeted T cells rapidly induce molecularremissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Science Translational Medicine 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930
[7] Tran E, Turcotte S, Gross A et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014;344:641–645. doi: 10.1126/science.1251102
[8] Morgan R, Yang J, Kitano M et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Molecular Therapy 2010;18:843–851. doi: 10.1038/mt.2010.24


