Alamy
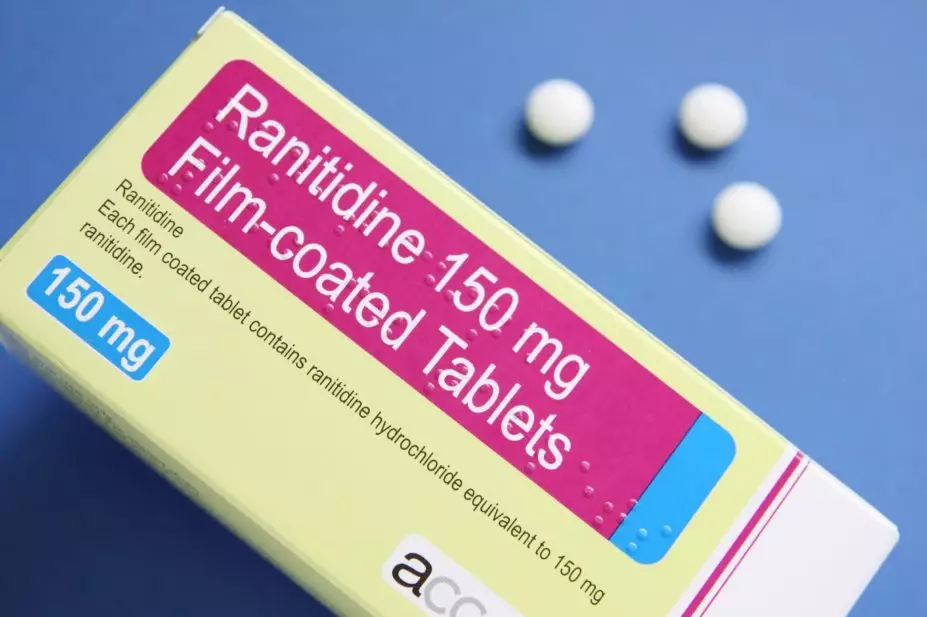
Medley Pharma has recalled unexpired stocks of ranitidine over possible contamination with N-nitrosodimethylamine (NDMA).
The Medicines and Healthcare products Regulatory Agency issued a drug alert, on 16 December 2019, recalling ranitidine 150mg and 300mg tablets from pharmacies and wholesalers as a precautionary measure.
Ranitidine medicines, which belong to a class of medicines known as histamine-2 blockers, are used to reduce the production of stomach acid in patients with conditions such as heartburn and stomach ulcers.
Medley Pharma is the 13th manufacturer to recall unexpired batches of ranitidine, with 33 such products recalled so far over possible contamination with NDMA – a probable human carcinogen.
In October 2019, GSK recalled four ranitidine products, Teva recalled 150mg and 300mg ranitidine effervescent tablets, Rosemont Pharmaceuticals recalled its ranitidine 150mg/10ml oral solution and Omega Pharma and Galpharm International recalled three of their ranitidine products — all over possible NDMA contamination.
The recalls continued in November 2019, with Creo Pharma and Tillomed Laboratories recalling one ranitidine product each. In the same month, OTC Concepts, Relonchem Limited, Noumed Life Sciences Limited and Medreich PLC recalled a total of 13 ranitidine products.
Earlier in December 2019, Accord Healthcare then recalled two of its ranitidine tablet products.
The recalls come after the European Medicines Agency (EMA) announced a review of ranitidine medicine in September 2019 to ascertain whether the NDMA risks patient health. Later that month, the EMA told drug manufacturers that all medicines containing chemically synthesised active substances should be tested for the presence of NDMA and other nitrosamines.