Shutterstock.com
Source: Shutterstock.com
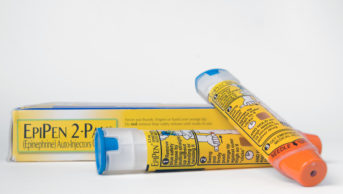
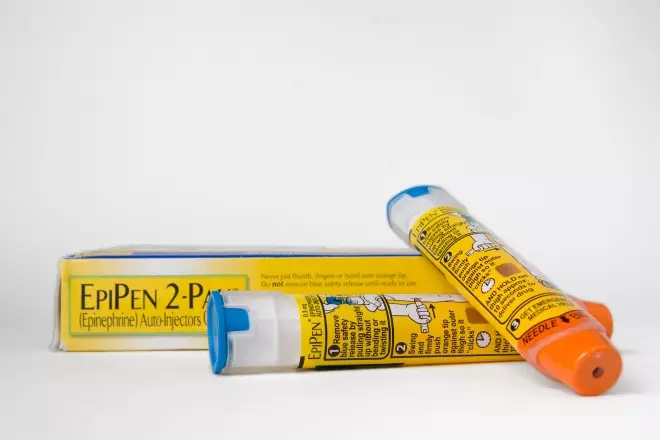
Coroner Sean Cummings said that to access muscle “the preferred needle length is 25mm for adrenaline injectors”, but the EpiPen’s needle length was 16mm
The coroner who investigated the death of Natasha Ednan-Laperouse has called on the Medicines and Healthcare products Regulatory Agency (MHRA) to take action after he said the EpiPen’s “inadequate dose of adrenaline for anaphylaxis and an inadequate length needle” raises serious safety concerns.
Ednan-Laperouse, aged 15 years, died on 17 July 2016 after suffering an anaphylactic reaction on a flight to Nice, France, having eaten a baguette bought from Pret A Manger at Heathrow Airport. Although her father administered two EpiPen injections, Ednan-Laperouse died in hospital a few hours later.
In his ‘Report to prevent future deaths’, Sean Cummings, assistant coroner for the Coroner Area of London (Western Area), said that to access muscle “the preferred needle length is 25mm for adrenaline injectors”. Cummings said that during the inquest, he heard that the EpiPen’s needle length was 16mm, its standard length.
“The use of needles which access only subcutaneous tissue and not muscle is, in my view, inherently unsafe. An alternative autoinjector, Emerade, has a 24mm needle,” he wrote.
Cummings also said that although the UK Resuscitation Council recommends a standard emergency adrenaline dose of 500mcg, the EpiPen contains 300mcg. Again, he compared EpiPen to Emerade, saying that the latter “contains a dose including 500mcg”.

Source: Shutterstock.com
Coroner Sean Cummings has called on Clive Schlee, chief executive of Pret A Manger, among others, to respond to his ‘Report to prevent future deaths’
In his report, Cummings says: “Action should be taken to prevent future deaths and I believe you, Clive Schlee, chief executive of Pret A Manger; the Medicines and Healthcare products Regulatory Agency; the chief executive of Pfizer; and secretary of state Michael Gove all have the power to take such action.”
The named parties have until 3 December 2018 to respond to the report. In a statement, Pfizer said: “We empathise deeply with the family on their loss. We always strive to cooperate fully with authorities, however in this case, we need to clarify that the questions posed by the coroner relate to the drug approval in the UK and therefore should be directed to the license holder.”
The Pharmaceutical Journal contacted Mylan UK for comment.
This article was amended on 15 October 2018, to reflect the fact that the coroner discussed adrenaline doses in micrograms not milligrams.