Shutterstock
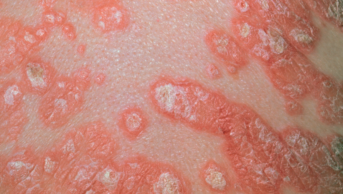
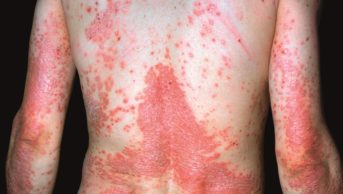
A new treatment for mild-to-moderate atopic dermatitis has been approved by the US Food and Drug Administration for use in patients aged two years and older.
Eucrisa (Anacor Pharmaceuticals, recently acquired by Pfizer) is applied topically twice daily. It is a phosphodiesterase 4 (PDE-4) inhibitor, although its specific mechanism of action in atopic dermatitis is not known.
The safety and efficacy of the ointment was established in two placebo-controlled trials involving a total of 1,522 patients, aged from 2 to 79 years, with mild-to-moderate atopic dermatitis. A greater response was seen in patients receiving Eucrisa, with this group of patients showing clear or almost clear skin after 28 days of treatment.
Serious side effects of Eucrisa include hypersensitivity reactions, and the ointment should not be used by anyone who has had a hypersensitivity reaction to Eucrisa’s active ingredient, crisaborole. The most common side effect of Eucrisa is application site pain, including burning or stinging.