GW Pharmaceuticals
A cannabidiol (CBD)-based medicine for severe epilepsy has been given marketing approval by the European Commission.
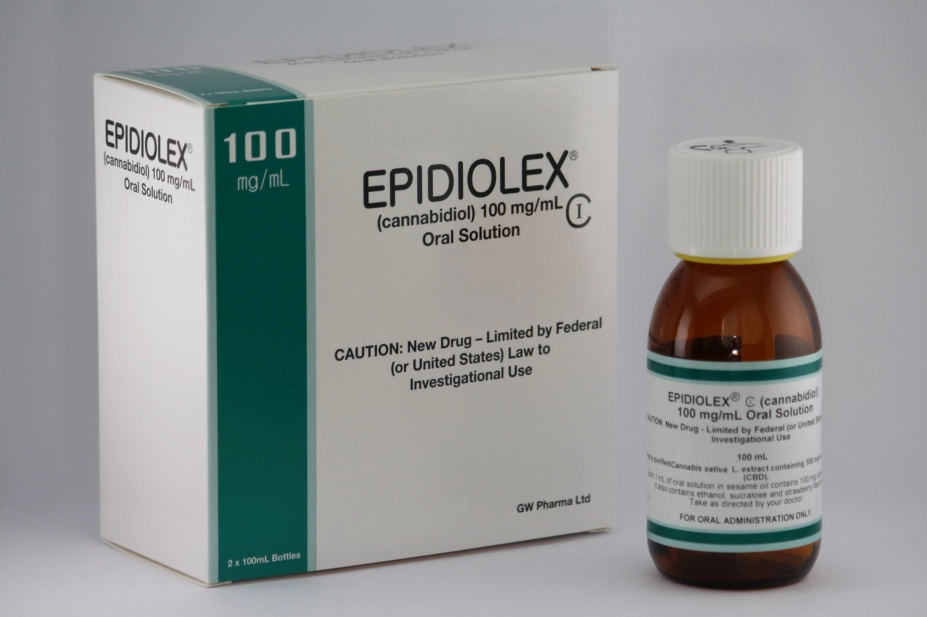
Epidyolex, manufactured by GW Pharmaceuticals, has been approved as an adjuvant treatment for seizures associated with Lennox Gastaut syndrome or Dravet syndrome in patients aged two years or over. It is to be used alongside clobazam.
The medicine is a 100mg/mL oral solution of cannabidiol. The only other cannabis-based medicinal product with similar authorisation is Sativex, an oromucosal spray for treatment of seizures associated with multiple sclerosis, which contains both CBD and delta-9-tetrahydrocannibinol.
The European Commission’s decision follows the European Medicines Agency’s recommendation in July 2019 that the medicine be granted full marketing authorisation. The marketing authorisation will apply across all 28 countries in the EU, as well as Norway, Iceland and Liechtenstein.
The US Food and Drug Administration gave its approval to the medicine, under the name Epidiolex, in June 2018.