Shutterstock.com
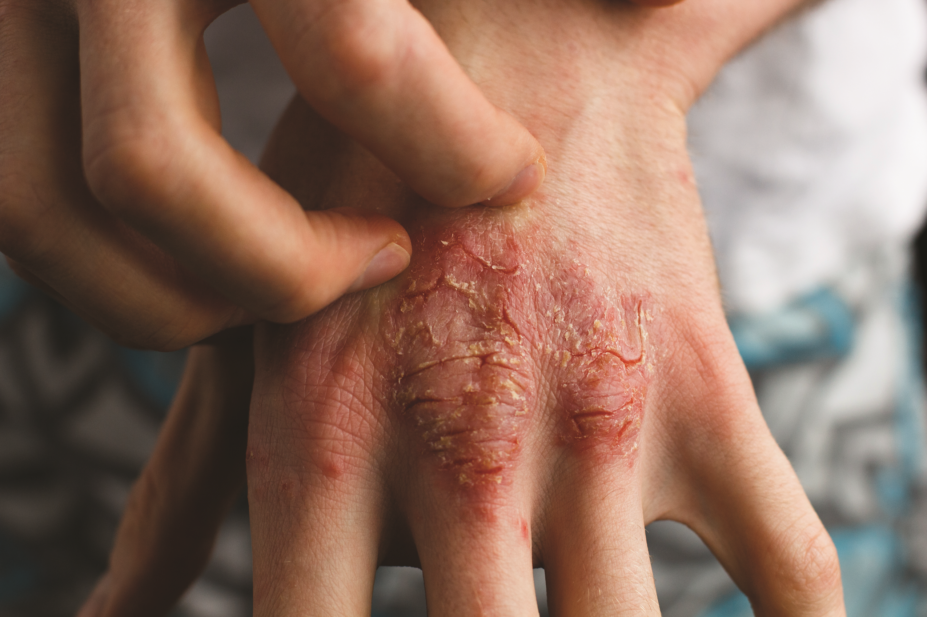
The US Food and Drug Administration (FDA) has approved an injectable biologic, brodalumab (Siliq), for psoriasis in adults that comes with a warning about the risk of suicide.
The monoclonal antibody, which is indicated for moderate-to-severe disease, will receive a boxed warning and will only be available through a risk-evaluation mitigation-strategy programme, due to the treatment’s association with suicidal ideation and suicide events in clinical trials.
During clinical trials, six patients treated with the drug committed suicide, a rate that was 3-4 times higher than that seen in other trials of biologics for psoriasis. The FDA said that although a causal link to suicide has not been established, the treatment should only be available through a restricted programme to try to minimise the risks.
It requires prescribers to counsel patients on the risks, and they must sign a patient-prescriber agreement to receive the drug. Pharmacies must also be certified with the programme in order to dispense it.
Brodalumab was developed under a collaboration agreement between Amgen and AstraZeneca. But in May 2015, Amgen abruptly ended their partnership on the drug, saying that the suicidal and behaviour events recorded during the trials would likely result in strict labelling requirements, limiting the potential patient population. Rights to the drug were subsequently licensed from AstraZeneca by New Jersey-based Valeant Pharmaceuticals.
Brodalumab works by targeting the interleukin-17 receptor and thereby preventing an inflammatory cascade that leads to psoriasis plaques. The drug’s efficacy was explored in three randomised placebo-controlled trials involving more than 4,000 patients where, according to the pharmaceutical company Valeant, more than half of all patients had complete skin clearance of psoriasis within a year.
The most common adverse reactions were headache, joint pain, fatigue, throat pain, and diarrhoea.
In Europe the drug will be commercialised by LEO Pharma. A marketing authorisation application for brodalumab was submitted in the fourth quarter of 2015 to the European Medicines Agency and is currently under review.


