Shutterstock
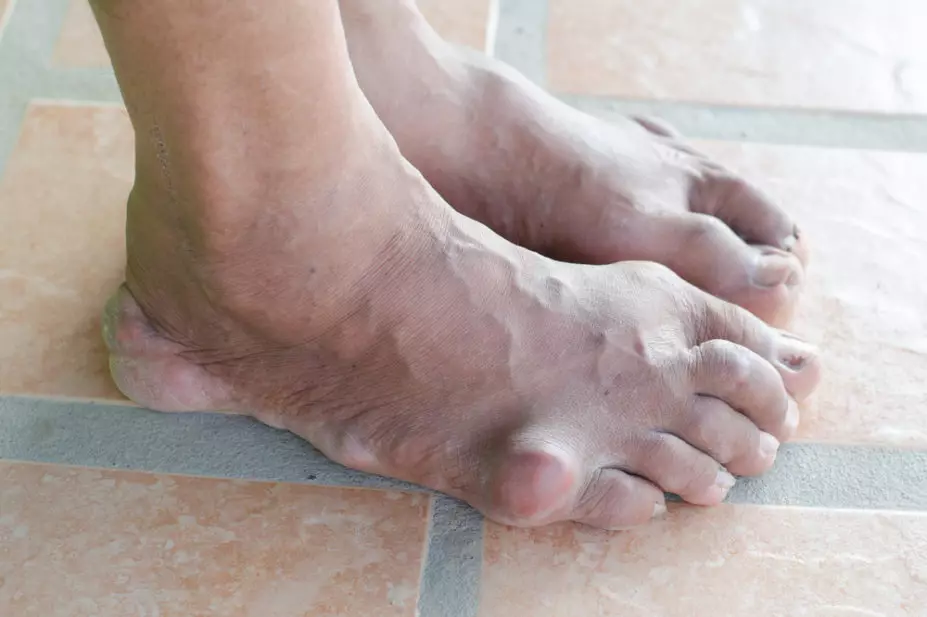
A new treatment for gout that blocks the reabsorption of uric acid in the kidneys has been approved by the US Food and Drug Administration (FDA).
Lesinurad, marketed as Zurampic by AstraZeneca, has been approved for use in combination with a xanthine oxidase inhibitor, such as allopurinal, which reduce the production of uric acid in the body.
Gout is the result of excess uric acid in the blood stream (hyperuricaemia), which can lead to the formation of uric acid crystals that cause pain and swelling in the joints, usually beginning in the big toe. Lesinurad helps the body excrete more uric acid by inhibiting a receptor called URAT1 in the kidneys, reducing the amount of uric acid that is reabsorbed. In three phase III clinical trials involving 1,537 patients, lesinurad reduced serum uric acid levels more than placebo in patients who did not reach their treatment targets with a xanthine oxidase inhibitor alone.
“Combination therapy with Zurampic is an important addition to the medicines available to … help more gout patients reach their serum uric acid treatment targets, which may ultimately relieve their suffering from this painful disease,” says Lawrence Edwards, chairman of the US Gout and Uric Acid Education Society.
Lesinurad is administered as a 200mg tablet. The most common adverse reactions in clinical trials were headache, influenza, increased blood creatinine and gastroesophageal reflux disease.
In Europe, the drug was given a positive recommendation by the European Medicines Agency’s Committee for Medicinal products for Human Use on 17 December 2015 and awaits final approval by the European Commission.


