Shutterstock
Revised warnings about the risks of being left permanently disabled after taking fluoroquinolone antibiotics have been added to products in the United States following a safety review by the US Food and Drug Administration (FDA).
The FDA carried out a review and found that both oral and injectable fluoroquinolones are associated with potentially lifelong disabling side effects involving tendons, muscles, joints, nerves and the central nervous system. The effects can appear weeks after taking the antibiotics.
The agency has subsequently decided that fluoroquinolones should be reserved for serious bacterial infections, including anthrax, plague and bacterial pneumonia where their benefits outweigh the risks.
For patients with acute bacterial sinusitis, acute exacerbation of chronic bronchitis and uncomplicated urinary tract infections, fluoroquinolones should only be used when there are no alternative treatment options, the FDA says.
The boxed warnings that appear on the packets of fluoroquinolone medicines have been updated by the FDA, drawing attention to the risk of disability and the new prescribing restrictions for specific patients. This information also appears in a patient medication guide, which must be given out with each fluoroquinolone prescription.
The new safety advice about fluoroquinolones is the latest by the FDA which first warned about the risks associated with this class of antibiotics in 2008.
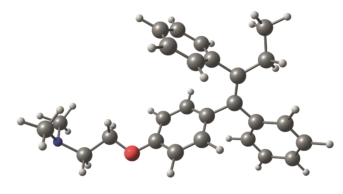
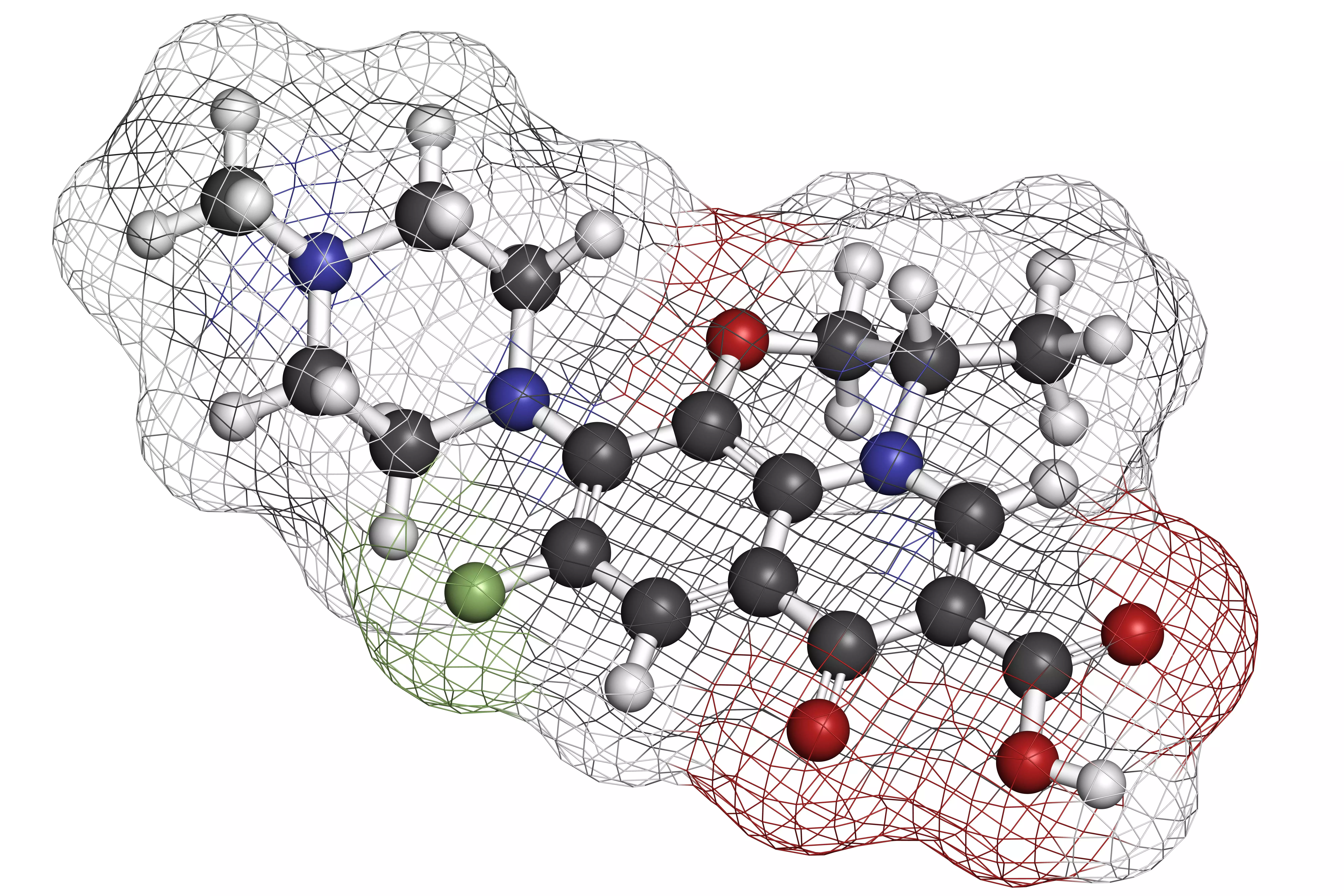
In the United States, FDA-approved fluoroquinolones include levofloxacin, ciprofloxacin, ciprofloxacin extended-release tablets, moxifloxacin, ofloxacin and gemifloxacin.