Introduction
Recently there has been an increased emphasis on patients playing an active role in decision-making about their care, and achieving concordance with prescribers around the use of prescribed medicines. This requires greater discussions around treatments and an expectation on the part of patients that they will be involved in making decisions about their care. However, this can be difficult for patients, particularly when the outcomes of treatment are uncertain or when the options available to them have benefit and harm profiles that they value differently.[1]
To help patients make various decisions about their health care, several decision aids have been developed which provide greater information in a format that is easy to understand.
A recent Cochrane review[2]
commented that decision aids improve knowledge and realistic expectations, enhance active participation in decision-making, lower decisional conflict, decrease the proportion of people remaining undecided, and improve agreement between patient values and choice. To date they have not been well evaluated in actually improving adherence. However, in some cases non-adherence may be the intentional result of a decision on the part of the patient not to take medicines or to take them in a way that differs from the clinician’s instructions.[3]
The comments of the Cochrane review would suggest that, despite not being designed to improve adherence, the decision aid might have a role in reducing intentional non-adherence by encouraging discussion, negotiation and education.
The average rate of compliance with bisphosphonates is 30 per cent and patients who are non-compliant are up to three times more likely to have a risk of fracture.[4]
We undertook a pilot study to assess the acceptability of a decision aid and its potential impact on patient adherence with oral bisphosphonate medication.
Methods
Subjects
Postmenopausal women prescribed oral bisphosphonates (in a single GP practice with eight partners) were invited by letter to participate if they had a diagnosis of osteoporosis confirmed by dual-energy X-ray absorptiometry (DEXA) or were aged over 65 years and had radiological evidence of fragility fracture. Patients prescribed oral bisphosphonates because of long-term steroid use were excluded. Patients who consented to be included in the study were randomised into a control group receiving normal care or an intervention group. Group allocation was done by a third party, unconnected to the study and blinded to the identity of the patients. The study was approved by the local ethics committee.
Outcomes
Patients’ views on the usefulness and acceptability of the workshop, decision aid and consultation were assessed using three open questions inviting their comments on aspects of the intervention.
Adherence or compliance, defined for this study as “the extent to which the patient’s behaviour in terms of taking medications coincides with the clinical prescription”,[5]
was measured by monitoring repeat bisphosphonate prescription requests, and expressing the actual number of prescriptions to the number expected assuming perfect adherence. The result was expressed as a percentage use by each patient. We also measured attendance at routine clinics and self-reported compliance using a standard tool, the Medication Adherence Report Scale (MARS) (R. Horne, personal communication).
Other outcomes were patient satisfaction, assessed using the Satisfaction with Information about Medicines Scale (SIMS),[6]
Beliefs about Medicines Questionnaire (BMQ),[6]
patient comments and a visual analogue scale, and, in the intervention group only, decisional conflict using the Decisional Conflict Scale.[7]
DCS scores of 2.0 or lower are normally associated with no difficulty in decision-making and behavioural implementation of decisions while scores of 2.5 or greater are associated with decision delay.[8]
At baseline in the intervention group only, a visual analogue scale was used to seek patients’ views on changing therapy. In the final questionnaire the extent to which decision-making was assisted by the intervention was assessed in the intervention group by asking patients to indicate whether they wished to remain on current therapy, change or were undecided.
Decision aid
The decision aid used was developed in Canada, where it is undergoing further evaluation of its effects on compliance.[9]
The aid comprises an information booklet, an audiocassette and worksheet to be used at home by the patient before an appointment with a doctor. The target audience is postmenopausal women with osteoporosis considering treatment options to prevent further bone loss and fractures. Literacy skills (Flesch-Kincaid Grade Level 5.8) and a moderate level of interactivity are required. The decision aid was adapted for a British context by the original Canadian team with assistance from the British researchers, overseen by the Medicines Partnership. This included modifying the language for a British audience, ensuring that the medicines mentioned were commonly available in the UK, rerecording the cassette tape with British voices and British patients’ experiences, and updating the information with the most recent evidence.[10],[11]
Intervention
Patients in the intervention group were invited to attend an osteoporosis workshop. In the workshops patients were introduced to the decision aid and the group discussed the worksheet. This involved working through the steps in the decision-making process identifying patients’ personal lifetime risk of a hip fracture, other personal or family health issues that might influence their decision, as well as self-care options they were already using or willing to try. Patients then considered their personal values regarding the pros and cons of prescribed therapies and what was important to them in making a decision. Any questions they had for the GP were noted on the worksheet and they recorded their preferences for being involved in deciding about therapy. Finally, women indicated which of the prescribed therapies they were leaning towards at that point.
Approximately two weeks later, the women returned with their completed worksheets for a follow-up consultation with one of the partners, a GP with a special interest in osteoporosis. (The patients on this GP’s personal list were excluded from the study. During the study period patients were only permitted to see this GP for the intervention consultation.)
Questionnaires were administered as shown in Figure 1 and data were extracted from practice records to establish compliance at baseline and four months post-intervention.
Compliance was reassessed in the intervention group four months after the GP consultation using the methods employed in the baseline assessment. The control group were also reassessed at this point.
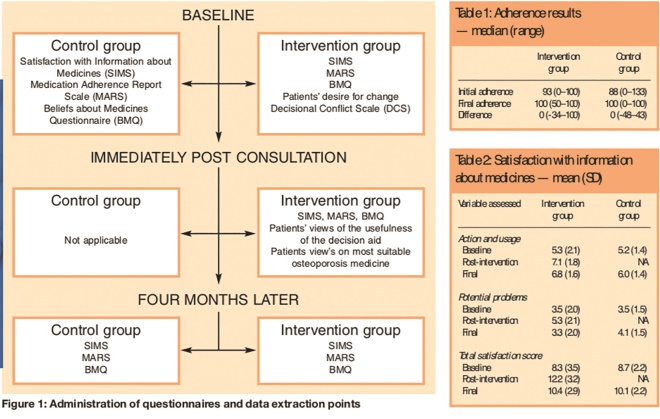
Figure 1: Administration of questionnaires and data extraction points; and Tables 1 and 2: Adherence results — median (range); and Satisfaction with information about medicines — mean (SD)
Results
Two hundred and forty-nine patients were prescribed oral bisphosphonates (213 female). Of these 110 patients satisfied the criteria for entry and were invited to participate. Only 33 accepted and were randomised to give 16 in the intervention group and 17 in the control group. No evaluation was carried out to determine the reasons for non-participation. The average age of patients was 77 years (range 61–90), with no differences between the groups. A similar proportion of patients in the intervention group (13/16) and control group (11/17) had previously experienced a fracture (P =0.44 Fisher’s Exact test).
Acceptability
Patients’ comments about the usefulness of the workshop, decision aid and GP consultation indicated that the consultation was the most valued element of the intervention but they expressed satisfaction with all elements.
Adherence
The difference in adherence improvement between the two groups (Table 1) is not statistically significant (P =0.80 Mann Whitney U test). Combining both groups overall, 12 patients did not change adherence, 12 improved and six got worse. However, this was not statistically significant (P =0.47 Wilcoxon Signed Ranks test). The patient in the control group who had not requested any medicines previously continued to be non-adherent, although a similar patient in the intervention group requested all expected medicines for 100 per cent compliance post-intervention. In contrast to these results, self-reported compliance using the MARS suggested that compliance was nearly perfect in both groups throughout the study (intervention 98 per cent, control 97 per cent). No patients stopped taking medicines. Only patients in the control group had requested more medicines than would have been expected before the study.
Patient satisfaction
Satisfaction with information about medicines improved in the intervention group immediately after the intervention but this gain was largely dissipated by the final questionnaire (Table 2). The change in total satisfaction score from baseline to final scores was not statistically significantly different between the two groups (P =0.80 Mann-Whitney U test).
Decisional conflict
This was assessed in the intervention group only. The results for the Decisional Conflict Scale indicated a degree of decisional conflict in all areas initially but post-intervention the degree of decisional conflict was reduced to a level where all patients would be expected to be able to make a decision about their medication (total DCS score pre-intervention, median (range), 2.5 (1.8–3.4) v 2.0 (1.0–2.4) post-intervention, P <0.001 using the paired Student’s t-test. Pre-intervention 13 per cent of patients scored 2 or less, with 53 per cent scoring 2.5 or more, but post-intervention 57 per cent scored 2 or less and none scored above 2.4. Similar changes were seen in each domain of the DCS scale. A high proportion of patients (93 per cent) expressed satisfaction with the choice they had made about their medication post-intervention.
At baseline, 25 per cent of patients in the intervention group indicated that they would consider changing therapy, and a further 50 per cent were unsure about whether or not they would like to change. In fact, two participants (12 per cent) did change to a different preparation (etidronate to risedronate) after their consultation with the GP. However, the mean values for satisfaction with medicine measured on the visual analogue scale remained fairly constant at 7.8 throughout the study in both groups. After the intervention 75 per cent of patients in the intervention group wanted to remain with their current therapy. No patients were undecided.
Discussion
In this study, most patients were highly satisfied with the intervention and decision aid tested. They particularly valued the opportunity to discuss their treatment with the GP in a dedicated consultation, but also the workshops because they were able to meet and discuss their problems with other women with osteoporosis. The GP involved in the consultation was reviewing a decision with the patient that had been made by one of his partners and although some changes were made no patients stopped taking medicines. The decision aid certainly improved participants’ ability to make a decision about which treatment was best for them. However, the results were less conclusive about the decision aid’s ability to improve adherence.
The randomisation process took no account of the range of bisphosphonates taken by the patients or the prescribing intervals for these products. In addition, prescription medicines requested may not necessarily have subsequently been collected, dispensed or taken by the patient. These may have been confounding factors.
The number of patients recruited to the study was small. Although the reasons for this were not formally investigated, GPs at the practice reported being contacted by patients invited to participate who were seeking advice on whether or not they should agree to take part. In general, younger patients are keener than older patients to be involved in treatment decisions,[12]
and the age of the participants in the study might have made them less keen on the intervention. It is also possible that these older patients did not wish to jeopardise their relationship with their GP and may have found the intervention more acceptable if they had been able to see their own GP.
Based on reanalysis of the adherence data using means (SD), improvement in adherence from baseline was 2.0 per cent (26.0 per cent) in the control group and 10.4 per cent (32.0 per cent) in the intervention group — a mean difference of 8.4 per cent and a pooled standard deviation of 29.0 per cent. With the sample size in this study there was 80 per cent power to detect a standardised effect size of 1.0 which, knowing the standard deviation, equates to a difference of 29.0 per cent. These results can be used to estimate the sample size for a larger trial. To have 80 per cent power to detect a difference of 8.4 per cent, as in this trial, would require 189 patients per group. To detect differences of 10 per cent and 20 per cent would require sample sizes of 133 and 34 per group, respectively. It would be important for anyone considering a larger study to consider the magnitude of difference that would justify the investment in time and resources required to use the decision aid in practice. Any future study might consider selecting only those patients with a low level of adherence for inclusion in the study.
Calls to the National Osteoporosis Society indicate that many patients diagnosed with osteoporosis do not feel they have received enough information from health care professionals.[3]
Even after the intervention tested here, sub-analysis of the results of the detailed medicines information profile in SIMS revealed that participants required more information in some areas, including how they could tell if their medicine was working. Many patients are reported to feel confused and anxious about the type of medication they have been prescribed for osteoporosis[3]
and this was confirmed by the baseline responses to BMQ, DCS and SIMS in this study. Nevertheless, this study found these older patents enthusiastic to be involved in making decisions about their treatment.
The small sample size makes it impossible to draw any definitive conclusions from this study. However, the role of the decision aid as a part of a multifaceted approach to improving adherence and supporting patients needs to be assessed. A Cochrane review has recommended that optimal strategies for dissemination of decision aids are explored.[2]
In this study patients appreciated the opportunity to discuss their treatment with the GP, and it would be useful to evaluate the extent to which the decision aid contributed to the success of this consultation. The GP certainly believed that the workshops and decision aid facilitated his discussions with patients. He considered his discussions with patients were on a “higher” level than would otherwise have been possible and expressed confidence in knowing that the patient had received their information from a reliable and unbiased source.
Non-adherence wastes resources and results in a missed opportunity for therapeutic benefit.[3]
Patient information leaflets, while useful, can be alarming and may be a cause of intentional non-adherence. Decision aids offer patients an opportunity to understand the probable benefits and risks of treatment and to participate actively with practitioners in deciding about medicines. They may have a role to play in reducing intentional non-adherence when used by pharmacists in a medicines use review or by other health care professionals in falls prevention teams or fracture clinics.
Acknowledgements
We thank: the prescribing support team at Poole Primary Care Trust and Alan Fisher and the staff at Westbourne Medical Centre; Geraldine Mynors and Caroline Kelham of the Medicines partnership; Rob Horne, of the University of Brighton for permission to use his validated questionnaires; Ann Cranney and her team at the University of Ottawa for permission to use the decision aid and the Decisional Conflict Scale; and Peter Thomas of Poole Hospital for advice on statistical analysis. Unrestricted educational grants to support this work were provided by Eli Lilly & Co Ltd, Merck Sharp & Dohme Ltd, and Strakan. The Medicines Partnership provided practical support and funded production of the decision aid.
This paper was accepted for publication on 8 March 2005.
Sue Oakley, MSc, MRPharmS, is pharmaceutical adviser to Poole Primary Care Trust, Westover House, West Quay Road, Poole Dorset BH15 1JF. Tom Walley, MD, FRCP, is professor of clinical pharmacology at the University of Liverpool.
Correspondence to Ms Oakley (e-mail sue.oakley@poole-pct.nhs.uk)
References
[1] O’Connor A, Edwards A. The role of decision aids in promoting evidence-based patient choice. In: Edwards A, Elwyn G (editors). Evidence-based patient choice — inevitable or impossible? New York: Oxford University Press; 2001. pp220–42.
[2] O’Connor AM, Stacey D, Entwistle V, Llewellyn-Thomas H, Rovner D, Holmes-Rovner M et al. Decision aids for people facing health treatment or screening decisions (Cochrane Review). In: The Cochrane Library, Issue 3, 2003. Oxford: Update Software
[3] Horne R. In: McGavock H (editor). Handbook of drug use research methodology: Newcastle: UK Drug Utilisation Research Group; 2000.
[4] Scottish Intercollegiate Guideline Network. Guideline number 71: The management of osteoporosis. Edinburgh: SIGN; 2003.
[5] Girvin B, McGavock H. In: McGavock H (editor). Handbook of drug use research methodology: Newcastle: UK Drug Utilisation Research Group; 2000.
[6] Horne R, Hankins M, Jenkins R. The Satisfaction with Information about Medicines Scale (SIMS): A new measurement tool for audit and research 12075. Quality in Health Care 2001;10:135–40.
[7] Horne R, Weinman J, Hankins M. The Beliefs about Medicines Questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychology and Health 1999;14,1–24.
[8] O’Connor A. Decisional Conflict Scale 2000. Available at: http://decisionaid.ohri.ca/eval.html (accessed 25 April 2006).
[9] Cranney AB, O’Connor AM, Jacobsen MJ, Tugwell P, Adachi JD, Ooi DS, et al. Development and pilot testing of a decision aid for postmenopausal women with osteoporosis. Patient Education and Counselling 2002;47:245–55.
[10] Chlebowski RT Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D et al, for the Women’s Health Initiative Investigators. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women. JAMA 2003; 289:3243–53.
[11] Million Women Study Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 2003;362:419–27.
[12] Cassileth BR, Zupkis RV, Sutton-Smith K, March V. Information and participation preferences among cancer patients.Annals of Internal Medicine 1980;92:832–6.
You may also be interested in

EMA may ask manufacturers to increase production capacity in response to medicines shortages

Meeting May 2024 Pharmacy First payment thresholds is ‘an area of risk’, warns Community Pharmacy England
