Abstract
Aim
To determine the effectiveness of a community pharmacist intervention to promote effective use of emollients in children with atopic eczema.
Design
Pre-post intervention pilot study.
Subjects and setting
Children aged 1–7 years with eczema and their parents currently using emollient bath oils, recruited through advertisements in local schools and playgroups and through the National Eczema Society. The intervention was delivered by community pharmacists in Brighton, Coventry and London.
Outcome Measures
Parent- or child-rated severity of itch; parent- or child-rated severity of sleep disturbance, irritability and skin appearance; emollient use (adherence); attitudes to eczema and emollient therapy.
Results
50 subjects completed the intervention and follow-up. 20% had previously been shown how to apply emollient creams by a GP or nurse but only 10% were applying them correctly. There was a small, statistically significant reduction in itch following the intervention (mean reduction 1.43, SD 2.76, t=3.61, P=0.001), a small, statistically significant reduction in irritability (mean reduction 1.23, SD 2.84, t=2.86, P=0.006) but little reduction in sleep disturbance or skin appearance. 44% of subjects experienced a 50% or more reduction in itch and irritability. Following the intervention, there was an increase in the number of subjects who applied emollient creams correctly.
Conclusions
A community pharmacist intervention to promote the effective use of emollient creams in children with eczema is effective and is valued by parents. The intervention increases the correct use and application of emollients (adherence to recommended use) and significantly reduces symptoms.
Atopic eczema is a common disease in children with a prevalence of around 20 per cent. It has a significant impact on the quality of life of children and their families. It affects all aspects of their lives, including sleep, school attendance and inclusion, play activities and emotional well-being.1–3 Recommended first-line treatment is with emollients and they are frequently prescribed. However they are often not used in an effective way even though using them effectively may reduce the need for potent topical steroids.4 The Primary Care Dermatology Society and British Association of Dermatologists Guidelines for the management of atopic eczema5 state that patients and parents of children with eczema should be fully informed (in the consultation and with written information) about the condition and treatment options and that the correct application of emollients should be demonstrated. However, the main reason many patients and parents report dissatisfaction with consultations with dermatologists and GPs is that they are given little information about the condition and treatments2 and less than 5 per cent of parents recall receiving any demonstration of how to use or apply topical treatments.6 There is evidence that educational interventions,7 including those provided by dermatology specialist nurses, significantly increase the effective use of emollients with a consequent 89 per cent reduction in symptom severity.4,6,8,9 However, few children with eczema have access to specialist nurses. Community pharmacists are in an ideal position to be a source of support and advice for the majority of children who are diagnosed by their GP and never see a dermatologist of specialist nurse, for several reasons:
- They are the health professionals most likely to have contact with the child or parent in the early stages of the condition
- They see them every time they pick up their prescriptions or buy an over-the-counter preparation for eczema
- With the introduction of the new pharmacy contract, many have facilities for consultations and are offering additional services
We undertook a pilot study to evaluate the feasibility and effectiveness of a simple intervention delivered by community pharmacists to promote the effective use of emollients by children with atopic eczema or their parents.
Methods
We undertook a pre-post intervention evaluation pilot study based in community pharmacies in Brighton, Coventry and London. This was a non-randomised, uncontrolled study to consider whether a simple pharmacist intervention, delivered within the scope of everyday practice, could promote the effective use of emollient creams and bath oils in children with eczema with a consequent improvement in eczema-related symptoms. The study was conducted over a four-month period from October 2005 to January 2006.
Participants
We recruited children aged between one and seven years with diagnosed atopic eczema and their parent(s). Eligible children were current or recent users of bath emollients that they received on prescription from their GPs. Subjects were recruited through advertisements placed in local schools and nurseries, and in participating pharmacies, and through the National Eczema Society. All subjects gave written informed consent.
Participating pharmacists were recruited from Brighton, Coventry and London to capture variation in patient populations. Pharmacists were identified through previous experience of or participation in research studies and through primary care trusts and included independent pharmacies and small chain pharmacies. Eligible pharmacists had to have access to a private consulting room or area.
All participating pharmacists received identical training for the study. This training was designed from materials provided by an eczema specialist nurse and included:
- Background information about eczema (prevalence, characteristics, symptoms and the range of treatments available)
- Specific information about the role of emollients in managing eczema
- Information about the problems some parents have reported about emollients
- A physical demonstration of the recommended way of applying emollients, in terms of the amount of emollient to be used, how to apply it (using light downward strokes) and that a thin layer should be visible on the skin
- Further information about how to apply emollients, ie, how often they should be applied, and that they should be applied to the whole body not just the affected areas
- What information and advice to give parents about overcoming specific problems they might encounter with emollients
- A guide to some of the common misperceptions about emollients and how to address these
- Specific guidance on good research practice, ie, obtaining informed consent and completing research paperwork
Training was conducted face-to-face with pharmacists within two weeks of the start of the study and each pharmacist was given a training manual which contained all the information given during the session.
Intervention
Eligible, consenting subjects were given an appointment to see a participating local pharmacist in the pharmacy. Pharmacists organised their own appointment slots to fit around times when the pharmacy was shut (for example, lunchtimes), during the quieter times in the pharmacy, or when there was adequate pharmacist cover within the pharmacy.
At the appointment, the pharmacist reviewed patient’s or parent’s management of eczema using a standardised proforma. This review included:
- Current treatment (bath and topical emollients, soap substitutes, topical steroids and any other product)
- Patterns of use of emollients (frequency, amount applied or added to the bath)
- A demonstration by the patient or parent of how he or she currently applied topical emollients
Following the standardised guidance provided by a dermatology specialist nurse, the pharmacists then gave advice about the correct use of bath and topical emollients with full explanations of the rationale underpinning correct use and practical tips for making effective use of emollients easier to achieve. They then demonstrated the correct method of application of topical emollients. Finally, they discussed concerns and questions raised by the patients or parents and gave them written information about eczema and treatment to take away.
The intervention took between 10 and 15 minutes to complete.
Data collection
We assessed participating subjects twice: up to one week before and one month after their pharmacist appointment. The assessments consisted of a standardised questionnaire administered over the telephone by an independent trained researcher. All subjects were assessed by the same researcher both before and after the intervention.
The questionnaire is specifically designed to capture detailed information on the use and experience of pharmacological treatments and identify barriers to their effective use. It also includes a measure of the impact of the medical condition on quality of life and the severity of symptoms. It is a generic tool that has been validated and used in a number of chronic diseases.10 The questionnaire captured:
- Information about the child’s eczema (duration, severity, attributed cause)
- What information or advice they had previously received and from which health professionals
- Current management of eczema (prescription products, non-prescription products, alternative therapies)
- Impact of the child’s eczema on family quality of life
- Use of emollients (frequency, amount applied or added to the bath, method of application)
- Perceptions of efficacy of current emollient therapy
- Barriers to the effective use of topical emollients
- Attitudes to eczema and treatment
- Concerns about topical emollients
Current severity of the symptoms (itch, irritability, sleep disturbance and skin appearance) were measured on a 0–10 scale where 0 represented no symptoms and 10 the worst.
The follow-up questionnaire was identical to the baseline questionnaire but with additional questions about contact with health professionals for advice or treatment for eczema within the follow-up period, and specific questions about the pharmacist intervention (what was covered and how helpful it had been).
Statistical analysis
We used descriptive statistics to describe the current management of eczema, use of emollients and attitudes and concerns about eczema and emollient treatment. We used paired t-tests to evaluate change in the primary outcome (symptom severity) before and after the pharmacist intervention. We used SPSS 13.0 to analyse all data.
Results
Fifty children and their parents completed the intervention and follow-up. Ten pharmacists delivered the interventions. The mean age of participating children was 3.8 years (SD 1.88, range 16 months to 7 years) and mean duration of eczema 3.0 years (SD 1.8, range 6 months to 6 years). Forty-two of the children (84 per cent) were Caucasian.
Symptom severity
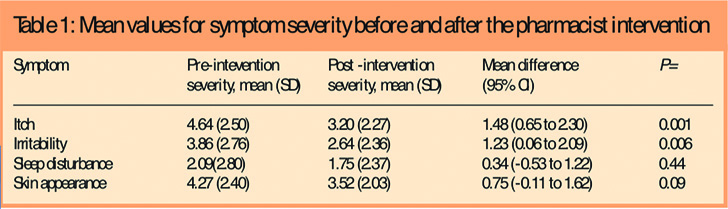
Seven children (14 per cent) were rated by their parents as having severe eczema, 12 (24 per cent) moderate eczema and 31 (62 per cent) mild eczema. The mean scores for symptom severity are given in Table 1. There was a small, statistically significant reduction in itch and irritability following the pharmacist intervention (Table 1). There was also a reduction in redness and scaliness of the skin following the intervention but this was not statistically significant. There was little reduction in sleep disturbance.
Current management of eczema
All children were currently using or had recently used an emollient bath oil prescribed by their doctor. In addition, many children were using either a soap substitute or emollient cream with or without a topical steroid. Some children were using several similar products, for example, more than one emollient cream. Twenty-six children (52 per cent) were using a topical steroid. In addition, five children (10 per cent) were having wet wraps to control their eczema.
Nineteen children (38 per cent) were on special diets to help manage their eczema but there was little use of alternative therapies: six (12 per cent) were taking a homoeopathic remedy, two (4 per cent) were using Chinese medicine and none was having acupuncture.
Effective use of emollient creams
Before the pharmacist intervention, few parents were using emollient creams as recommended to maximise efficacy. For example:
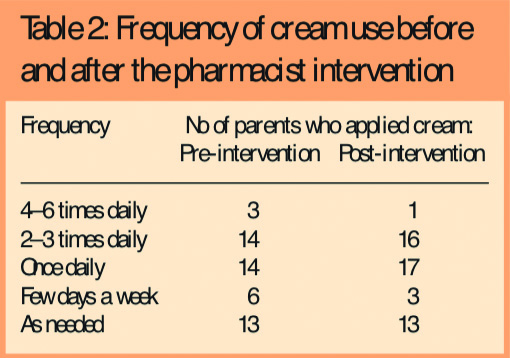
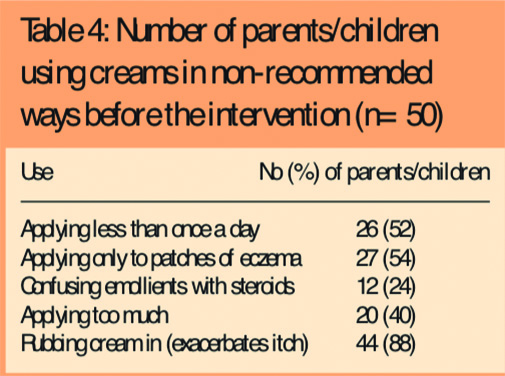
- Only 17 (34 per cent) were applying creams more than once a day; 26 (52 per cent) were applying them less frequently than once a day (Table 2)
- Two-fifths (20, 40 per cent) were applying the creams so as to leave a thin layer on the surface of the skin as recommended (Table 3)
- Around half (27, 54 per cent) were applying the creams just to the patches of eczema instead of the whole body.
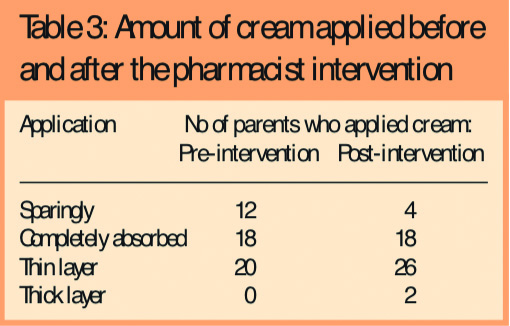
There was evidence that some parents (12, 24 per cent) were either confusing emollient creams with steroids or following the same advice as for topical steroids and applying them sparingly.
Pharmacists observing parents’ application of cream to their children reported that only around half (27, 54 per cent) of parents were using the correct amount of cream, 20 (40 per cent) were using too much, and only six (12 per cent) used the correct method of downward strokes in the direction of hair growth. Forty-four (88 per cent) were rubbing the cream in, which can irritate the skin and exacerbate the itch.
Following the intervention, there was some change in parent-reported use and application of emollient creams in line with recommended practice, ie, an increase in adherence to recommended use (Tables 2 and 3) and 31 parents (62 per cent) were applying cream to the whole body rather than just the eczema patches. There was also a reduction in the number of parents applying emollients as topical steroids from 12 (24 per cent) to four (8 per cent).
Barriers to use of emollient creams
Several potential barriers to the effective use of emollient creams were identified. Twelve parents (24 per cent) reported that their children did not like having the cream applied, 19 (38 per cent) found them time-consuming to use and 28 (56 per cent) said the creams made a mess of clothes, furniture and bed sheets.
Beliefs and concerns about eczema and treatment
Twenty-two parents (44 per cent) believed their child had inherited their eczema from a family member, 11 (22 per cent) thought their child had developed eczema because of an allergy to something such as cow’s milk and 10 (20 per cent) thought it had been caused by pollution.
Most parents (42, 84 per cent) believed that the health of their children’s skin depended on treatment with emollient creams, but many (32, 64 per cent) were worried about having to use the creams over prolonged periods and around half (26, 52 per cent) were concerned that their children might become dependent on treatment for their eczema.
Previous advice and information from healthcare professionals
Most children (49, 98 per cent) had seen their GP for advice and treatment for their eczema. Fifteen (30 per cent) had seen a dermatologist and eight (16 per cent) had seen a dermatology specialist nurse. Thirty children or parents (60 per cent) had received a verbal explanation of eczema and the prescribed or recommended treatment from the health professional(s) they had seen, 16 (32 per cent) had received written information and advice and 10 (20 per cent) had been shown how to apply the topical emollient prescribed.
Patient/parent feedback on the pharmacist intervention
Most parents (39, 78 per cent) rated the pharmacist intervention as extremely helpful or quite helpful. Those that rated it as only a little helpful or not helpful said that the intervention itself had been useful but that they had not learnt anything new because they had extensive experience of managing eczema through having several affected children.
Discussion
We have demonstrated that a simple intervention to help parents and children to manage eczema more effectively through the correct use of emollient creams can be delivered by community pharmacists within their pharmacies and that this intervention is valued by parents. We have also demonstrated a small, statistically significant reduction in eczema symptoms following the intervention, although because this is a non-randomised, uncontrolled pilot study in a small number of subjects and pharmacists, this result should be interpreted with caution.
In this study we found that many parents were applying emollient creams ineffectively and that few (10, 20 per cent) had ever been shown how to apply them correctly. This highlights a gap in knowledge and understanding that may be having an impact on disease outcome. Emollient creams and bath oils are recommended as a first-line, ongoing treatment for atopic eczema and it has been suggested that effective use of emollients may be steroid-sparing.3 Using them effectively therefore, may be important in reducing the amount of steroid treatment children are exposed to. Previous studies have demonstrated that dermatology specialist nurses are extremely effective in educating people with eczema about their condition and in particular, how to use treatments such as emollients,5,6 but few children have access to specialist nurses. In this study, only 16 per cent had seen a dermatology specialist nurse. We need to consider which other health care professionals may have a role in providing information and advice to parents.
Community pharmacists are ideally placed to fulfil this role. Almost all parents and children will have access to a pharmacist when they collect their prescribed emollient and pharmacists have the expertise to review treatment and understand the potential problems as well as often seeing what other non-prescription products parents are using to manage their child’s eczema. One issue in undertaking an intervention such as this in a busy pharmacy will be time. This intervention took between 10 and 15 minutes and that time may not always be available at the time when parents collect prescriptions. However, the new community pharmacy contract may provide an opportunity to undertake interventions such as this, for example as a medicines use review.
Interpretation of this study is limited by its size and design. It was primarily designed as a pilot study to examine whether an intervention delivered in a community pharmacy could have an impact on the use of emollients in eczema and to identify any aspects of the intervention or its delivery that might require modification before its adoption and assessment in clinical situations. The effectiveness of such interventions when delivered by specialist nurses has already been demonstrated. Although we found a statistically significant reduction in symptoms following the intervention, we cannot say conclusively that the reduction was a direct result of the intervention rather than a change in symptoms that reflects the patterns of exacerbation and improvement in symptoms that occur in the natural history of eczema, or a chance finding. In addition, our small group of pharmacists may not be representative of pharmacists more generally in their ability to provide interventions such as this. The pharmacists involved in this study had previous research experience of delivering interventions or had been providing additional professional services for some time. This may mean that they were more experienced than other pharmacists in delivering interventions. The subjects were self-selected and may have been more likely to be responsive to an intervention of this type than other parents of children with eczema. Both these situations would have promoted a positive result in this study. However, if such an intervention were to be introduced into routine clinical practice, it is likely that the pharmacists most likely to adopt it would be similar to our group and that subjects would to some extent be self-selecting in the same way as they were in this pilot study.
To show consclusively that an intervention delivered by community pharmacists results in symptom improvement would require an adequately powered randomised controlled trial in a larger number of subjects and with a greater number of pharmacists delivering the intervention to take account of the variability in pharmacist practice. What we have shown is that community pharmacists can deliver interventions to provide information and advice to parents of children with eczema, that they can do so within their usual practice and that such an intervention is highly valued by parents. Further work is needed to demonstrate the effectiveness of this intervention in changing behaviour, promoting adherence and concordance over a longer period, reducing symptoms/improving control and minimising the need for topical steroids. We also need to examine how interventions in eczema can be best incorporated into the community pharmacy contract within additional or enhanced services.
Acknowledgements
We are grateful for the hard work of all the other pharmacies involved in the study: J. Goes; G. Gohi; M. Bridgman; R. Bhasin; Vantage Chemist, Hayes, Middlesex; Daya Ltd, Hayes, Middlesex.
Declaration
This study was funded by Stiefel Laboratories. All research was undertaken by Clinamatrix, an independent clinical research company. The data were analysed by the authors. Stiefel had no access to the raw data and took no part in the management of the study.
This original paper was accepted for publication on 9 February 2007.
About the authors
Alison Carr, PhD, is special lecturer in musculoskeletal epidemiology at the University of Nottingham and research director at Clinimatrix.
Rohit Patel, BSc, MRPharmS, Mandy Jones, BSc, MRPharmS, and Abdul Suleman, BSc, MRPharmS, are community pharmacists in Hayes, Middlesex.
Correspondence to: Dr Carr at Clinimatrix, Hale House, Hail House Lane, Churt, Surrey GU10 2JQ (e-mail alison.carr@wgclinimatrix.co.uk)
References
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. British Journal of Dermatology 1991;16:333–7.
- Long CC, Funnell CM, Collard R, Finlay AY. What do members of the National Eczema Society really want? Clinical and Experimental Dermatology 1993;18:516–22.
- Moore K, David TJ, Murray CS, Child F, Arkright PD. Effect of childhood eczema and asthma on parental sleep and wellbeing: a prospective comparative study. British Journal of Dermatology 2006;154:514–8.
- Holden C, English J, Hoare C, Jordan A, Kownacki S, Turnbull R et al. Advised best practice for the use of emollients in eczema and other dry skin conditions. Journal of Dermatological Treatment 2002;13:103-6
- Primary Care Dermatology Society and British Association of Dermatologists Guidelines for the management of atopic eczema. 2006;28:372–5.
- Cork MJ, Britton J, Butler L, Young S, Murphy R, Keohane SG. Comparison of patient knowledge, therapy utilization and severity of atopic eczema before and after explanation and demonstration of topical therapies by a specialist dermatology nurse. British Journal of Dermatology 2003;149:582–9.
- Staab D, Diepgen TL, Fartasch M, Kupfer J, Lob-Corzilius T, Ring J et al. Age related, structured educational programmes for the management of atopic eczema in children and adolescents: multicentre, randomised controlled trial. BMJ 2006;332:933–6.
- Broberg A, Kalimo K, Lindblad B, Swanbeck G. Parental education in the treatment of childhood atopic eczema. Acta Dermato-Venereologica 1990;70:495–9.
- Ersser S, Latter S, Surridge H, Buchanan P, Satherley P, Welbourne S. Psychological and educational interventions for atopic eczema in children. Cochrane Database of Systematic Reviews 2003;(1):CD004054
- Carr AJ, Hughes RA, Vincent K, Carr M, Thwaites C. The impact and implications of new treatments in arthritis: validation of a questionnaire to measure the “real life” effectiveness of medication. Rheumatology 2004;43(Suppl):ii83.


