Abstract
Aim
To identify a packaging design that is acceptable (easy to open and retrieve medicine) to patients with inflammatory arthritis and establish patient preference for packaging designs.
Design
Cross-sectional observational study in which patients evaluated seven different packaging designs, including a blister pack and a standard child-resistant container.
Subjects and setting
103 patients with inflammatory arthritis attended a single session in the outpatient department at St Peter’s Hospital, Chertsey, Surrey.
Outcome measures
Primary outcome was the packaging acceptability score, a composite score from a patient-completed questionnaire based on ease of use, ease of retrieval and preference.
Results
The child-resistant packaging and the blister pack performed significantly worse than any of the other designs, receiving the worst ratings from patients (80% of patients rated the child-resistant bottle as the worst packaging). 48% of patients said they would decant medicines from a child-resistant bottle into another container that was easier to use. There was a clear preference for one design of packaging that was the highest rated design by 41% of patients. Preferences for design were independent of hand pain, function, grip strength or hand deformity.
Conclusion
Child-resistant packaging and, to a lesser extent, blister packs can be difficult for people with inflammatory arthritis to open. This presents a potential hazard because 48% of patients would decant medicines from the child-resistant bottles into other containers that may not have appropriate labelling. Alternative designs of packaging are available that conform to British Standards for medicines packaging, are easy to open for people with impaired hand function and may help to reduce medication errors and give patients more control over their medication.
Rheumatoid arthritis (RA) is the most common inflammatory arthritis, affecting more than 350,000 people in Britain.1 The effects of RA and other inflammatory arthritic diseases such as psoriatic arthritis and juvenile idiopathic arthritis vary widely from person to person, and may be minimal, or severe and debilitating. Hand function, including grip strength, is a common area of disability in subjects with inflammatory arthritis, adversely affecting the patient’s ability to access medicines from standard packaging.
Oral methotrexate has been used for many years as an effective therapy for moderate to severe RA and psoriatic arthritis, and is usually taken orally once a week. At the right frequency and dose, methotrexate is a safe medicine. However, over a period of 17 years to 2000, methotrexate was implicated in the deaths of 25 patients and in 26 cases of serious harm requiring admission to hospital.2,3 Medication errors were implicated in a number of these cases. Although many medication errors occur in prescribing or dispensing, some occur when the patient takes an incorrect dose or confuses medicines.
In 2003, the National Patient Safety Agency (NPSA) undertook an investigation into how the packaging and labelling of medicines — and particularly that of methotrexate — can influence patient safety (personal communication). Of the 12 subjects taking oral methotrexate assessed in their preliminary study, most experienced severe difficulties with opening medicines containers, particularly those with child-resistant closures and, to a lesser extent, blister packs.
The study confirmed that inadequacies in the packaging of medicines can indirectly compromise patient safety as patients adopt coping strategies to overcome both the inconvenience in retrieving medicines and even the pain that can result through the accessing of tablets. Coping strategies include decanting medicines from their original containers (or blister outer packs) into incorrectly labelled or unlabelled containers4,5 and failure to close child-resistant tops, compromising the integrity of the tablets.6,7
The NPSA study was small and only a limited number of observations were collected. The authors suspected that methotrexate users in the wider community might experience even greater problems than those found in their study and recommended strongly that further research be conducted.
The aim of this study was to evaluate packaging for methotrexate in a study population who would be eligible for treatment with this drug and who might also experience problems with hand function: patients with RA and psoriatic arthritis.
Methods
A cross-sectional observational study was undertaken in which patients with inflammatory arthritis evaluated seven different designs of medicines packaging. The evaluations were conducted in June and July 2006. The study was reviewed and approved by a local research ethics committee and hospital research committee.
Participants
Patients with inflammatory arthritis were recruited through the rheumatology outpatient clinics at St Peter’s Hospital and through the nurse practitioner database records of patients in active follow-up. Eligible participants were aged over 18 years and had a diagnosis of RA or psoriatic arthritis. All participants gave written informed consent.
Recruitment was structured to ensure that the sample included patients with and without hand deformities and that it captured a full range of hand functions.
Study procedures
Each participating patient attended a single study session held in the outpatient department at St Peter’s Hospital.
At the start of the session, their hands were clinically examined for deformities, a joint count (number of swollen joints and tender joints) was performed and grip strength was measured (three readings on each hand) using a Jamar hand dynamometer. They completed questionnaires to measure hand function and pain, and gave information about their current arthritis treatment, any co-morbidity that might affect hand function and medicines they were taking currently.
Patients then tested seven different packaging designs by attempting to open the packaging, retrieve the substitute medicine (selected to be as close as possible to the size of methotrexate tablets) and reclose the packaging.
The designs tested were a standard push down and turn child-resistant closure, a blister pack, and five designs of various sizes and with different styles of closure all of which are currently available and have been designed with the aim of increased ease of use for patients with some form of functional impairment (Figure 1).

The order in which the packaging designs were tested was randomised to ensure that one particular type of packaging was not always being evaluated first or last. The packaging to be tested was provided to subjects without any additional explanation or instruction beyond what would be enclosed with or written on the packaging in normal practice.
At the end of the session subjects completed a second pain questionnaire.
To provide some baseline for how patients manage to open their medicines packaging at home, subjects were asked to bring their usual medicines with them, together with any “tools” that they usually use to open the containers.
Subjects who gave specific consent were video-recorded while testing the packaging designs. This was to examine the different ways patients adapt to reduced hand function.
Packaging evaluations
Evaluation of the packaging designs was performed to British Standard EN ISO 8317:2004 and included time taken to open each design, time taken to retrieve substitute medicine from each design and time taken to reclose each design (for blister packs this entailed replacing the blister strip in the box).
Once subjects had successfully opened the package, retrieved the medicine and reclosed the package, they were asked to repeat the exercise as quickly as possible. After assessment of each packaging design, subjects rated the ease of opening, ease of retrieval and ease of closure on a questionnaire (packaging acceptability questionnaire).
To investigate and establish the potential impact that each design could have on patient safety, subjects were specifically asked whether they had ever decanted medicines from a dispensed packaging into a packaging of their choice. If so, they were asked how they then identified the contents of the alternative packaging. For each packaging design, they were asked whether for everyday use they would prefer or need to decant medicines from that package.
Once they had completed all evaluations, subjects were asked to give an overall, relative ranking/preference for each packaging design.
Questionnaire evaluations
Hand function
Hand function was assessed using a validated questionnaire (the Hand subscale of the AIMS28 questionnaire). AIMS2 is an internationally recognised questionnaire that is used in many RA clinical trials. It has been extensively validated and widely published. It is a short, self-completed questionnaire.
Hand pain
Current hand pain was quantified using a 100mm visual analogue scale. This is an accepted method of assessing pain in patients with arthritis and is widely used as a standard pain measure in clinical trials. This was completed on two occasions: immediately before the packaging tests and on completion of the study session.
Packaging ratings and preferences
Patient ratings for ease of use for each packaging design and their overall relative rankings and preferences were captured on the packaging acceptability questionnaire.
The questionnaire was designed specifically for this study and assessed for face validity, comprehensibility, ease of completion and acceptability in a pilot study before the full study. The questionnaire uses 10-point graphic rating scales to rate ease of opening, retrieval of medicine and closure for each packaging design and provides relative rankings for each design.
Outcomes
Primary outcome
Packaging acceptability score — a composite score obtained from the packaging acceptability questionnaire based on ease of use, ease of retrieval and preference.
Secondary outcomes
(1) For each packaging design, the proportion of subjects who achieved the five-minute and one-minute tests specified in British Standard EN ISO 8317:2004. That is: the proportion of subjects who were able to open and retrieve medicines from the packaging within five minutes on first attempt and the proportion of subjects who were able to open and retrieve medicines from the packaging within one minute when asked to repeat the exercise as quickly as possible. (2) Patient preference for packaging designs obtained from the packaging acceptability questionnaire. (3) Differences in acceptability and preference across different patient subgroups: hand deformity vs no hand deformity, and pain vs no pain.
Sample size determination
To ensure that the packaging design identified from this study is acceptable to patients across all severities of hand impairment, the study required adequate power to compare acceptability scores in patients with and without hand impairment.
In order to detect a clinically relevant standardised difference of 0.7 in packaging acceptability score with 90 per cent power, significant at the 5 per cent level, a minimum of 80 subjects (40 in each group) was required for analysis. To allow for drop-out, 103 subjects were recruited.
Statistical analysis
All data were entered in an SPSS database and analysed by one of the authors (AJC). Descriptive statistics were used to describe the demographic and clinical backgrounds of subjects and the ways in which they managed their medicines packaging at home.
Performance of each of the packaging designs was assessed by calculating mean (and 95 per cent confidence interval) packaging acceptability scores and time taken to access medicines and compared with other designs using paired t-tests. Comparisons of packaging acceptability between subject subgroups (hand impairment, no hand impairment, pain and no pain) were made using correlational analyses and Student’s t-tests as appropriate to test the null hypothesis of no difference between the groups. Data were analysed using SPSS for Windows version 13.0.
Results
Sample
One hundred and three patients were recruited and participated in the study: 75 (73 per cent) were female and 101 (98 per cent) were Caucasian. The mean age of the sample was 62 years (SD 11.09, range 41–92). Ninety-three patients (90 per cent) had RA, and 10 (10 per cent) had psoriatic arthritis. The duration of their arthritis ranged from one to 53 years (mean 18 years, SD 11.11). Three subjects had neurological co-morbidities (neuropathy) that affected their hand function.
Hand impairment
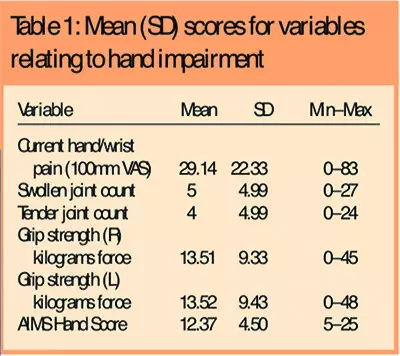
Patients represented a broad range of hand impairment related to pain, grip strength, swollen and tender joints and function. Table 1 shows the mean scores for all hand impairment variables.
Forty-eight patients (47 per cent) had some form of hand deformity that included muscle wasting (53 per cent), ulnar deviation (34 per cent), subluxation (36 per cent), boutonniere (14 per cent), swan neck (13 per cent) and fixed flexion (3 per cent).
Managing current medicines packaging
Sixty-six subjects (64 per cent) were taking methotrexate either alone or in combination with other disease modifying antirheumatic drugs, including anti-TNF alpha therapy. Seventy-one (69 per cent) had some of their arthritis medicines dispensed in child-resistant packaging and 95 (92 per cent) had some medicines dispensed in blister packs. Eleven subjects (11 per cent) routinely decanted their medicines into containers that had been used for other prescription medicines because of problems opening the packaging in which it had been dispensed and 20 (19 per cent) routinely decanted their medicines into some other (non-medicine) container for the same reason.
Forty-seven subjects (46 per cent) were able to open their medicines packaging without any help (from a person or implement) but usually only once the factory seal had been broken or removed.
Packaging evaluations
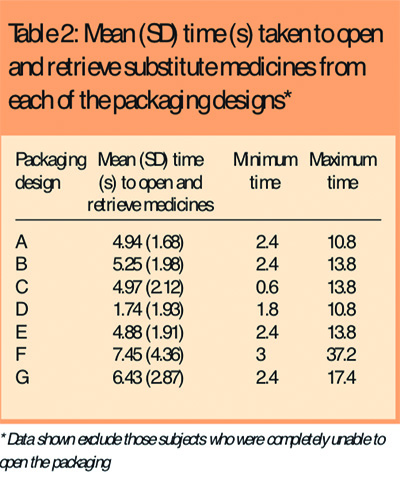
Most subjects were able to open the different packaging designs within a few seconds although it took slightly longer for them to open the child-resistant (G) and blister packaging (F) than any of the other designs. Six subjects (6 per cent) were unable to open the child-resistant packaging and three subjects were unable to open the blister packaging. One subject was unable to open any of the bottle packaging and could only manage the blister pack which she opened with a sharp fingernail that she kept long for precisely this purpose. Table 2 shows the mean time taken to open each of the packaging designs. Data from those subjects who were unable to open the packaging were excluded.
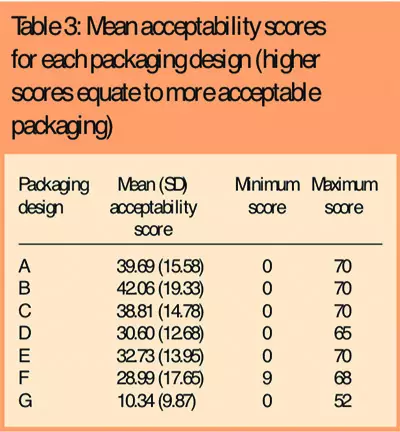
There was a clear preference among subjects for one packaging design (B), which attained the highest packaging acceptability score (Table 3) and was ranked as the best packaging design by 42 subjects (41 per cent) (Table 4).
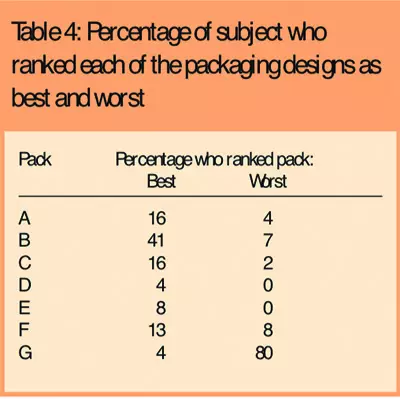
Reasons given for ranking this as the best packaging design were that the bottle size was easy to hold and the shape and size of the lid was easy to grip. The wide neck also made it easier to remove the medication. The child-resistant packaging (G) performed worst, with the lowest packaging acceptability score, and was ranked the worst packaging design by 82 subjects (80 per cent). The main reason given for ranking this as the worst packaging was that the push-and-turn movement required was difficult to perform, particularly when hands were painful.
Forty-nine subjects (48 per cent) reported that they would decant medicines from a child-resistant package like G, either because they would need to (n=16, 16 per cent), or because they would prefer to have their medicines in a container that is easier to use (n=33, 32 per cent). Around 20 per cent of subjects said that they would prefer to decant medicines from all the packaging designs tested, although this preference was more to do with the convenience of using calendar containers divided into sections for each day of the week which also provided reassurance that they had not forgotten a dose.
Effect of hand impairment on packaging evaluations
The performance and preferences for packaging designs were the same across subgroups of subjects with and without hand deformity and with and without hand pain, with B the preferred design and G the worst design. The child-resistant packaging (G) performed even less well in subjects with poorer hand function (Spearman’s rho –0.31, P=0.001) and weaker grip strength (Spearman’s rho –0.31, P=0.002) than in other subjects.
Discussion
We have evaluated the ease of use and patient preference for seven different packaging designs among patients with inflammatory arthritis. Standard child-resistant packaging that drugs such as methotrexate and many other arthritis drugs are dispensed in is difficult for patients with inflammatory arthritis to open and was rated as the worst packaging design by most patients in this study. The small number who did not rate it as the worst packaging said that they valued the packaging precisely because it was so difficult to open and they therefore felt that it was safe.
Difficulty accessing medicines in this sort of packaging represents a potential hazard because half the patients in this study said they would decant their medicines from this packaging into something that was easier to open but which may not be appropriately labelled or child safe. Confusion over dosing (both the frequency and the strength of the tablet) has been implicated in up to 52 per cent of the medication errors with methotrexate9 and the risk of this sort of error is likely to be increased if medicines are decanted from their original container. We found several instances where patients would leave child-resistant packaging partially open (and therefore no longer child-resistant) so that they could access their medicines. These findings support the results of the Cambridge study4 and indicate that these are fairly common behaviours among people with inflammatory arthritis.
Many subjects also experienced difficulty in opening and accessing medicines in blister packs (another commonly used packaging design for arthritis medicines) and this was rated the second worst design (after the child-resistant packaging). Of the other designs, there was a clear preference for one particular design (B) that was a wider bottle (easier to hold), with a large diameter top with undulations (easier to grip and turn) and a wide neck (easier to retrieve medication). Neither this, nor any of the other designs preferred by patients in this study, are child-resistant which highlights the conflict between making medicines accessible to the patients who need to take them and keeping them inaccessible to children. However, the results from this study and others suggest that many medicines are regularly stored in containers that are not child-resistant so that they can be accessed by patients, or that child-resistant containers are not locked shut to enable easier access. It may therefore be safer if medicines are dispensed in containers that are easy for patients to use and stored in areas inaccessible to children so that, rather than being decanted, the medicines can be kept in their labelled container, reducing the potential for dosing errors. This may be of particular importance where the dose has changed or where a different brand is dispensed.
Regulatory factors may influence the extent to which methotrexate and other arthritis medicines can be packed in patient-accessible containers by the manufacturer. Another limiting factor may be the relative cost and availability of the sorts of containers we tested in this study.
In some instances the problem of accessibility is being overcome within individual pharmacies. A few patients in this study reported that their pharmacist decanted the medicines into more accessible packaging for them at the time it was dispensed. The advantage of this is that the medicines are decanted safely and appropriately labelled. If the problems of accessible packaging can be overcome for patients with arthritis and any other condition that affects hand function, medication errors may be reduced and patients will have more control over their medicines.
Acknowledgements
The study was funded by an educational research grant from Pfizer Inc. The authors retain complete control over the design and execution of the study, the collection and analysis of the data and the interpretation and presentation of the results.
This paper was accepted for publication on 1 November 2007
About the authors
Rod Hughes, MD, FRCP, is consultant rheumatologist, Maggie Carr, MSc, DipNurs, is consultant nurse in Rheumatology, and Maggie Walsh is rheumatology research associate, at Ashford and St Peter’s Hospital Trust, Chertsey, Surrey. Alison Carr, PhD, is special lecturer in musculoskeletal epidemiology, Nottingham University.
Correspondence to: Dr R. A. Hughes, Department of Rheumatology, Ashford and St Peter’s Hospital Trust, Guildford Road, Chertsey, Surrey KT19 0PZ (e-mail Rod.Hughes@asph.nhs.uk).
References
- Arthritis Research Campaign. Rheumatoid arthritis. Available at: www.arc.org.uk/about_arth/booklets/6033/6033.htm (accessed 5 July 2005).
- National Patient Safety Agency. Patient Safety Alert 3: Preventing drug dosing errors with methotraxate. London: NPSA; 2004.
- National Patient Safety Agency. Patient Safety Alert 13: Improving compliance with oral methotrexate guidelines. London: NPSA; 2006.
- Report to the Committee on Safety of Medicines from the Working Group on Labelling and Packaging of Medicines. London: Medicines Control Agency; 2001.
- Canadian Public Health Association. Good medicine for seniors: Guidelines for plain language and good design in prescription medication. Ottawa, Ontario: CPHA; 2002.
- Mawle R. Which pill when: packaging that aids compliance in taking medication [thesis]. London: Royal College of Art; 2003. 7
- Clark P . An ergonomic evaluation of prescription medicine packaging and labeling for theelderly [thesis]. London: London University; 2002.
- Meenan RF, Mason JH, Anderson JJ, Guccione AA, Kazis LE. AIMS2. The content and properties of a revised and expanded Arthritis Impact Measurement Scales Health Status Questionnaire. Arthritis & Rheumatism 1992;35:1–10.
- Moore TJ, Walsh CS, Cohen MR. Reported medication errors associated with methotrexate. American Journal of Health-System Pharmacy 2004;61:1380–4.