Abstract
Aim
To describe a system of regular intervention monitoring among paediatric patients and how the information is used to inform the content of different strategies aimed at improving prescribing practice.
Design
Pharmacist Interventions were recorded using an intervention form. The design was based on the 2006 Pharmaceutical Care Network Europe (PCNE) classification scheme. Subcategories for paediatric pharmacy use were developed and all interventions reviewed by a second pharmacist to ensure the validity of the results. A Microsoft Access database was developed for data collection and analysis.
Subjects and setting
All paediatric inpatients at the Royal London Children’s Hospital were included. The hospital has 130 beds covering a wide range of tertiary specialties.
Results
In total 377 interventions were recorded over a six-month period. Most interventions (58 per cent) were due to dosing problems, where patients were dosed incorrectly for weight, age and indication. Errors in drug choice triggered 25 per cent of interventions and drug use 13 per cent. Within the latter two categories 41 per cent were caused by incorrect drug history reconciliation. Most interventions were deemed minor in severity, with 38 per cent classed as moderate and less than 1 per cent as major errors.
The data collected informed the content of different strategies to improve prescribing practice, including intervention bulletins, tailoring of doctor induction and a prescribing test and training for pharmacy staff.
Conclusion
The intervention form is a tool that is easy to use, which can provide valuable data to guide educational strategies and increase awareness of a variety of prescribing errors.
Prescribing errors are a significant risk issue in healthcare and a wide range of error rates are documented in the literature, ranging from 2 per cent to 14 per cent of medicines orders.1 One contributing factor is that the definition of a prescribing error varies significantly among studies.
One of the most commonly used definitions is that developed by Dean et al: “A clinically meaningful prescribing error occurs when, as a result of a prescribing decision or prescription writing process, there is (1) an unintentional reduction in the probability of treatment being timely and effective or (2) an increase in the risk of harm when compared with generally accepted practice.”2
There is some evidence from the Us that prevalence of medication errors could be higher in children3 and a recent paediatric study in the UK reported an overall prescribing error rate of 13.2 per cent.4 The higher error rate in paediatrics may not be surprising, considering the complexities of dose calculations in children and frequent use of unlicensed or off-label drugs. studies into prescribing errors have identified a number of contributing factors, such as lack of knowledge of the drug, its dosing and patients’ details, and inaccurate drug history taking.5
It has been recognised that pharmacists play an important role in mitigating the occurrence of errors,6 and in hospital are able to intervene at ward level as well as educate people and improve systems to guide safe prescribing.
Method
A Medline and Embase search was carried out in 2008 to search for validated methods of collecting studies about pharmaceutical interventions using the following search terms (limited to all children aged 0–18 years matched to thesaurus terms):
- “Hospital pharmacy service”
- “Pharmacists”
- “Medication errors”
The 2006 Pharmaceutical Care Network Europe (PCNE) classification scheme v5.01 for drug-related problems (DRP) was chosen to be adapted for use at The Royal London Children’s Hospital. This classification scheme is hierarchical and differs from other systems in that it separates the problems from the causes.
The following definition is the basis for the classification: “a drug-related problem is an event or circumstance involving drug therapy that actually or potentially interferes with desired health outcomes.”7
An intervention form was designed to ensure ease of data collection. The intervention form allowed details of the intervention to be documented by the pharmacist, and the categories to be analysed were set out in a quick tick-box format. The categories used for analysis of the drug-related problems were:
- Inpatient or discharge medicine (TTA)
- Problem
- Cause
- Harm
- Outcome
Appropriate subcategories for paediatric pharmacy use for cause of the drug-related problem were developed. Information on the consultant, ward, date and pharmacist reporting the intervention was also recorded.
A user guide was written, including any definitions of significance. The definitions of significance were in line with the trust incident-reporting system (Datix) definitions available at the time of development. The intervention-recording system was introduced and the user guide circulated to all team members and any new members joining the team.
A database using Microsoft Access was developed in-house to allow easy data collection and analysis. The data were input using dropdown menus that increase the validity of data entry. These also enable ease of analysis of the data captured since the data could easily be manipulated to show various results.
The intervention form is reviewed by the paediatric pharmacy team at least once a year to fine-tune the subcategories and any other recommendations to the latest version 3.
The significance of interventions made has always been contentious. On reviewing the intervention form at the beginning of 2012 a modification was made to divide the significance into “actual harm” and “potential harm”.
Pharmacists were finding it difficult to differentiate the significance of the intervention. For example, an overdose of paracetamol for one dose is categorised as “no harm” or “low harm”, but if the dose were continued in the long term it could cause hepatic damage, which is major harm. With the new actual and potential harm categorisation the overdose can be classified as “no actual harm, but potential moderate harm”.
All interventions were reviewed by a second pharmacist to increase the validity of the results. This pharmacist was one of the senior pharmacists who have been involved in the development of the intervention-recording project. He or she checked the categorisation of harm to ensure they agreed with the assessment of level of harm.
If the second pharmacist did not agree with the categorisation of harm the intervention was discussed with the pharmacist who recorded the intervention. A third opinion was sought when agreement cannot be reached.
The paediatric team recorded all interventions daily, including those for prescriptions to take home (TTAs).
The pharmacy department carried out annual intervention audits on interventions made one day a year.
Since the paediatric team launched the intervention-recording project, other teams have been inspired to collect daily interventions. The paediatric team helped redesign the intervention forms and individualised databases.
Results
From October 2011 to April 2012, six months’ data were analysed. February data have been omitted from this data set because paediatric wards were moved from the old hospital building to a new building and the low incident reporting and reduced clinical workload is deviates significantly from the expected results.
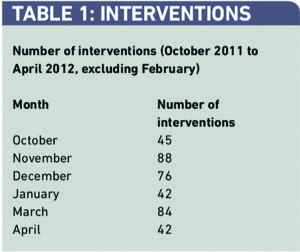
A total of 377 interventions were recorded over the six-month period by five or six pharmacists per month, depending on rotational numbers (Table 1).
Most interventions were problems associated with dosing, which presented itself on 217 occasions (25 per cent) as problems with drug choice (Table 2).
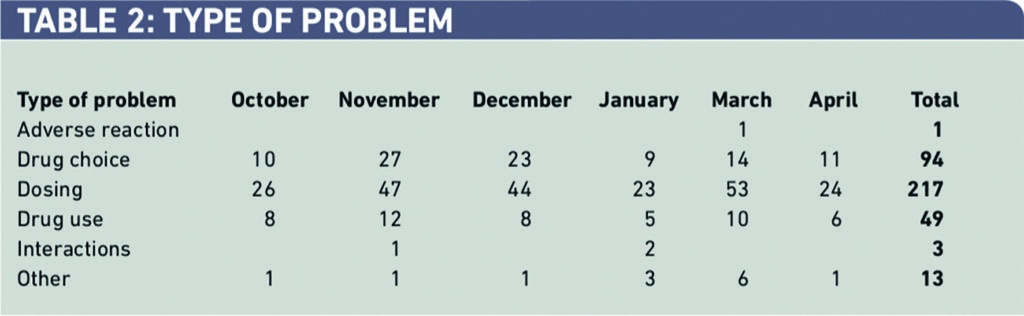
Within drug choice and drug use (143 interventions), 58 (41 per cent) were caused by incorrect drug history reconciliation with 23 (16 per cent) caused by missing transcriptions from the drug chart (Table 3).
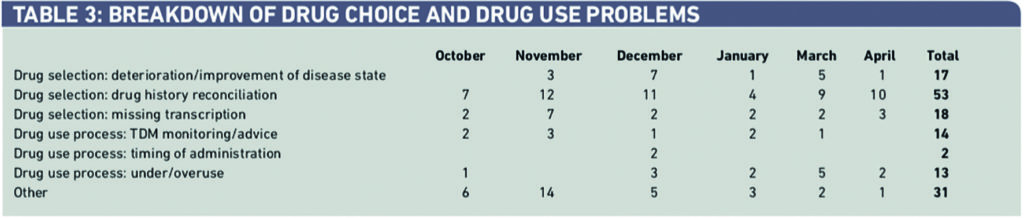
Within dosing problems, 27 per cent of 217 were dosed incorrectly for weight, 22 per cent for age and 21 per cent for the wrong indication (Table 4).
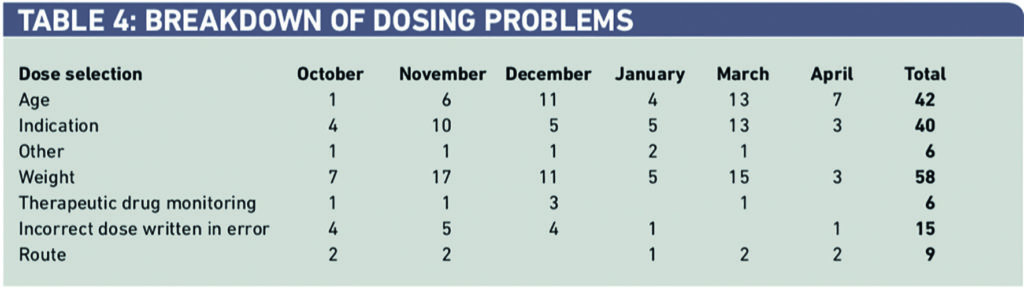
Sixty-two per cent of interventions were deemed minor in severity, with 38 per cent classed as moderate and less than 1 per cent as major errors and reported as an incident as defined by trust guidelines (minor — small improvement of patient care, moderate — significant improvement in patient outcome, major — life saving/prevents patient morbidity) (Table 5).
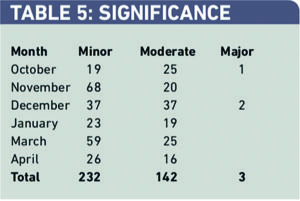
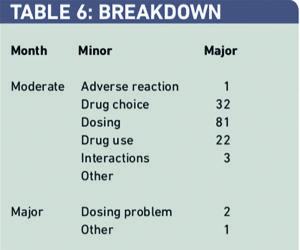
There were 142 major and three moderate interventions over the six-month period. Two out of three major interventions were dosing problems. Of the 142 moderately significant problems 81 (57 per cent) were dosing problems, 32 (23 per cent) were drug choice problems and 22 (15 per cent) were drug-use problems (Table 6).
Most interventions (138; 97 per cent) were completely solved, while the remainder were partially solved or the outcome was unknown. There were only three incidences where the problems were not solved by the pharmacist’s intervention.
Most interventions (316; 84 per cent) occurred on inpatient drug charts. The remainder were on discharge prescriptions.
Discussion
The number of interventions was not consistent over six months, ranging from 42 in one month to 88 in another, and the total number of interventions reported per person over the six-month period ranged from 27 to 120.
Benefits
The data collected were used to inform the content of different strategies to increase awareness among doctors, nurses and pharmacists around prescribing errors and to guide education and prescribing training. Different strategies were chosen based on practicality and affordability, with the aim of involving all the different healthcare professionals.
Intervention bulletins
Monthly intervention bulletins are sent out to all paediatric consultants and ward sisters for dissemination to their teams to increase awareness around prescribing issues. Initially this was done as a summary document for the whole of paediatrics, outlining how many errors occurred due to, for example, wrong dosing, inappropriate choice of drug, interactions or therapeutic drug monitoring as well as giving examples of particularly common or severe errors. Recent changes in data collection now enable the error rate to be traced to specialty level, thus enabling consultants to understand problems in their specific area better and guide their junior colleagues.
The dissemination of the information on pharmacists’ input to patient care also highlights their valuable contribution in reducing medication errors. Although no cost-avoidance data were collected, recognition of the pharmacist input into the care of the patient seems pertinent in times of increased pressure to cut staff.
Junior doctor induction and prescribing tests
In past years the time dedicated to prescribing training for new doctors at the Royal London Children’s Hospital has increased. The intervention data have informed which areas to focus on during the initial training and particular attention is given to drugs with which we see the highest error rates.
Based on the data, a prescribing test has also been developed and incorporated in the new doctor induction programme. This is not the Royal College of Paediatric and Child Health test but the scenarios are similar.
Education and training of pharmacy staff
Interventions are discussed monthly in the paediatric pharmacy team meetings and particularly interesting interventions are highlighted.
This often stimulates lively discussions and enables more junior staff to learn from the experience of others.
Pharmacists studying for a diploma find the interventions are an easy way to collect evidence for their portfolio and they can also be used to monitor individual pharmacists’ clinical work.
Limitations
Anecdotally, the number of interventions reported for discharge prescriptions is significantly under-reported, which misrepresents the number of prescribing errors made.
A reason for under-reporting may be the time constraint of a single pharmacist validating discharge prescriptiosns after the morning pharmacist visit. Also the categories for data input are dependent on the person entering the data, although error is reduced by the use of dropdown menus. The validity of interventions has been improved through review by a second pharmacist. Involving a prescriber in the interventions validation process would be even more desirable and we are currently looking at establishing this.
Different strategies have been used to improve prescribing based on the intervention data. From the data collected so far, it is not possible to ascertain the impact these different strategies had on reducing error rates. That will be the focus of future research.
Currently, no economic data are available to align the interventions we make with health economic worth.
Conclusion
In conclusion, the intervention form is a tool that is easy to use that can provide valuable data to guide educational strategies and increase awareness of types of prescribing errors.
About the authors
Nanna Christiansen is lead clinical pharmacist in paediatrics at Barts and The London Children’s Hospital.
Alice Lo is a highly specialist pharmacist in women’s and children’s risk at Barts and The London Children’s Hospital.
Andrew Law is an oncology pharmacist at Bupa Home Healthcare.
Correspondence to: Alice Lo (email Alice.lo@bartshealth.nhs.uk)
References
- Lewis PJ, Dornan T, Taylor D, et al. The prevalence and incidence of prescribing errors: Systematic Review, GMC report, 2008
- Dean B, Barber N, Schachter M. What is a prescribing error? Quality & Safety in Health Care 2000;9:232–7.
- Kaushal R, Bates DW, Landrigan C, et al. Medication errors and adverse drug events in pediatric inpatients. Journal of the American Medical Association 2001;(285):2114–20.
- Ghaleb MA, Barber N, Franklin BD, et al. Systematic review of medication errors in pediatric patients. Annals of Pharmacotherapy 2006;40:1766–76.
- William DJP. Medication errors. Journal of the Royal College of Physicians Edinburgh 2007;37:343–6.
- Dornan T, Ashcroft D, Heathfield H, et al. Final report. An in depth investigation into causes of prescribing errors by foundation trainees in relation to their medical education. EQUIP study. London: General Medical Council, 2009.
- Pharmaceutical Care Network Europe Foundation. PCNE Classification for Drug related problems V5.01, revised 1 May 2006. Available at: www.pcne.org/sig/drp/ documents/PCNE%20classification%20V5. 01.pdf (accessed 29 July 2013).