Abstract
Aim
To evaluate the efficacy and safety of two applications, seven days apart, of either isopropyl myristate/cyclomethicone solution (IPM/C) (Full Marks Solution) or permethrin 1% (Lyclear Creme Rinse) for the treatment of head louse infestation.
Design
Two assessor-blind, randomised, controlled, parallel group, clinical trials.
Subjects and setting
Community based with home visits involving 168 participants (141 children aged 1 to 17, and 27 adults) with active head louse infestation.
Outcome measures
Treatment success was defined as cure of infestation (ie, no evidence of head lice after the second treatment) or reinfestation after cure.
Results
Cure (or reinfestation after cure) occurred in 82.0% (91/111) of participants treated with IPM/C and 19.3% (11/57) of participants treated with permethrin 1% (P<0.001, difference 62.7%, 95% confidence interval 50.2% to 75.2%). IPM/C was found to be easier to apply to the hair and scalp (P<0.001), and had less odour (P<0.001). There was no significant difference between the products in terms of frequency or nature of adverse events.
Conclusion
IPM/C cures head louse infestation with a 10-minute contact time. It has a physical action that kills head lice that should not be affected by resistance to neurotoxic insecticides.
Head louse infestation is common in the community, with school-aged children being the most frequently affected. Recent studies found infestation in 8.3 per cent of 2,793 children (aged 4–11 years) in Wales,1 and 8.9% of 6,169 children (aged 2.5–12 years) in Ghent, Belgium.2
Resistance to insecticides was first observed in head lice in the UK in the early 1990s. It plays an important role in determining the effectiveness of the most popular head louse treatment products.3,4 Recent evidence has shown that the combing method of treatment, known as “bug busting”, is only successful at curing about half the people using it and there is a need for more effective treatments for this infestation.5,6 Full Marks Solution (isopropyl myristate/cyclomethicone solution, IPM/C) is a new treatment for head lice that uses a 10-minute contact time.
We have performed two randomised controlled trials, analysed together by intention to treat, comparing the efficacy of two applications, seven days apart, of IPM/C with permethrin 1 per cent (Lyclear Creme Rinse), the only other product in the UK that has a 10-minute treatment time.
Methods
Participants
Participants were recruited through newspaper and radio advertising throughout Cambridgeshire. Families with current head louse infestation contacted the study team for more information, an information booklet was sent to them by post, and those wishing to enrol subsequently telephoned to request a home visit. This process could be completed in a minimum of 24 hours and normally within 72 hours; as most potential participants had been infested for several weeks or months, time to first visit was not a deterrent factor for the majority. At the home visits, investigators, following a standard procedure, confirmed the presence of head lice using a plastic detection comb (“PDC”, KSL Consulting, Denmark). If lice were found, the person was invited to join the study and a signed consent and assent procedure was followed. The infestation level at baseline was estimated using the definitions in Panel 1.
Panel 1: Infestation level at baseline
- Light infestation: lice only found after five or six combs of the hair
- Moderate infestation: single louse found on the first comb of the hair
- Heavy infestation: more than one louse found on the first comb of the hair
However, if large numbers of lice were found during post-treatment checks and the level of infestation recorded at baseline was therefore considered to be incorrect, it could be reclassified at the final assessment. In practice, this rarely occurred, had no effect on study outcome or interpretation, and was used only to provide an estimate of how the products performed against different levels of infestation. Other household members with lice were invited to join the trial if eligible. Treatments and assessments were conducted in the home. No payment was offered for participation. Ineligible household members with an active head lice infestation (three people, see Figure 1) were offered a standard of care treatment — phenothrin 0.5 per cent liquid (Full Marks Liquid, SSL International).
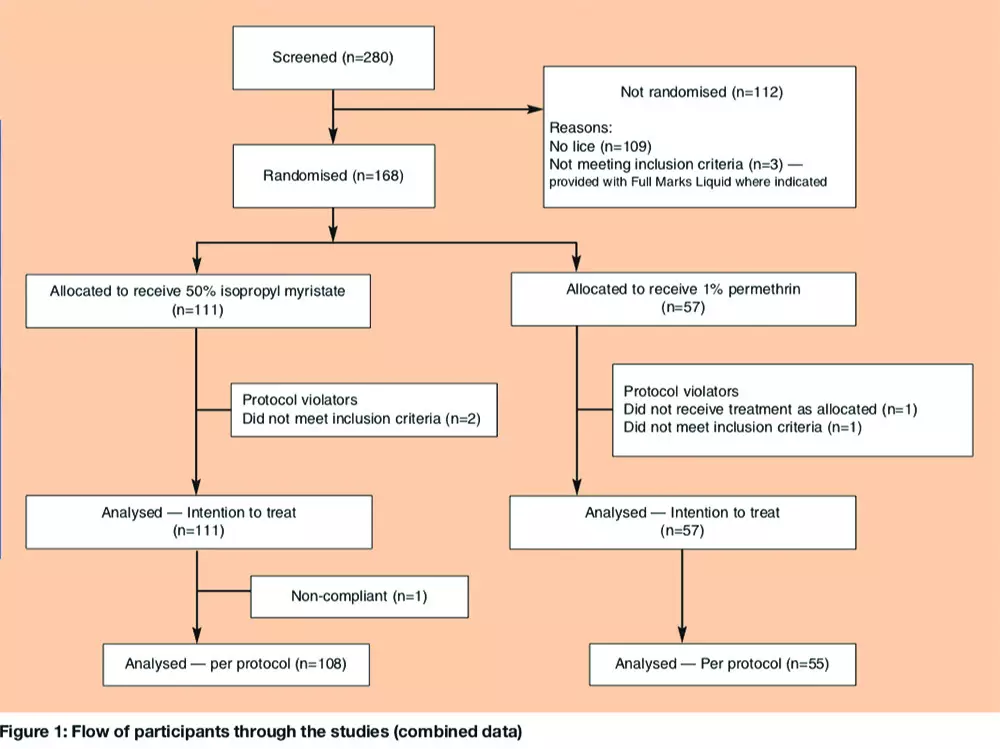
All enrolled participants provided baseline data of age, hair characteristics, and previous pediculicide use. A lower age limit of four years was set for Trial A. By the time of the second trial, however, sufficient experience had been obtained using IPM/C in the first trial and from post-marketing information for it to be considered acceptable to reduce the entry age to six months. There was no upper age limit in either trial.
For trial A, exclusion criteria were: pregnancy, breastfeeding, sensitivity to pyrethroid insecticides or chrysanthemums, chronic scalp disorders, pediculicide use within the previous two weeks, trimethoprim or co-trimoxazole use at evaluation or during the previous four weeks, recent use of hair bleaches, dyes, or permanent wave products, and participation in another clinical trial within one month.7 Trial B used the same exclusion criteria except that anyone with a chronic scalp disorder was permitted to participate so that any adverse effects in that sector of the population could be evaluated. (In practice, no such participants entered the study.) Other aspects of study design were identical and similar to those in an earlier study conducted by us.8
Interventions
IPM/C was supplied in 60ml (trial A) or 100ml (trial B) bottles (Full Marks Solution, SSL International, Manchester) and permethrin 1 per cent in 59ml bottles (Lyclear Creme Rinse, Chefaro UK Ltd, Huntingdon). IPM/C was applied to dry hair and was washed out after 10 minutes using shampoo and water. Permethrin was applied to freshly washed, towel dried hair and rinsed out after 10 minutes using water only. Sufficient of either product was applied to thoroughly coat the hair and scalp; more than one bottle could be used where required. Because there was no restriction on the quantity that could be used on each participant the different bottle sizes used for IPM/C, and the difference in size of these from the permethrin bottle, were not considered likely to influence outcome. In all cases the bottles were weighed before and after use so that the quantity used on each person could be calculated. Following application of either product, the hair was combed through with a normal grooming comb to spread treatment evenly and ensure thorough coverage. Treatments were applied to the full hair length. The same regimen was repeated seven days later. Participants were provided with 30ml bottles of non-medicated, conditioner-free shampoo as the pre-wash for permethrin and for removing IPM/C. Neither treatment included combing to remove lice or their eggs. Participants were asked not to use louse combs or other treatments during participation. Compliance with the protocol was assessed by retrospective questionnaire at each assessment.
Objectives
Trial A was designed as a proof of concept study with the aim also of comparing the efficacy of IPM/C with that of permethrin creme rinse. Trial B was designed to provide more information on safety of IPM/C as well as efficacy. In this case a dilemma was posed by the choice of comparator. Options were: (a) a placebo, which was generally considered unethical; (b) the permethrin comparator used previously, on the basis that the posology was essentially similar; or (c) some other marketed product with a different posology. In the event, and after discussion with the ethics committee, we decided that we would continue with the permethrin 1 per cent product, despite its low efficacy, because of the similarity in posology. However, on ethical grounds we reduced the number of people exposed to what was likely to be a less effective product and gave reassurance that they would receive phenothrin 0.5 per cent liquid, a treatment shown previously to be as effective as any on the market, as a rescue treatment should the permethrin fail. On that basis, and with the agreement of the local research ethics committee statistical adviser, randomisation was skewed in favour of IPM/C in a ratio of 4:1. As it happened, trial B was terminated early, after enrolment of 94 participants, for commercial reasons. At the time of the studies, medical devices for use against head lice were limited in the UK to products that removed head lice through a physical means, eg, combing. The sponsor terminated the study early because it was decided to continue registering IPM/C as a medical device rather than as a medicinal product. The decision to terminate the study was not based on safety or efficacy concerns, was made without interim data analysis, and was based entirely on the intended regulatory route for the product. The Medicines and Healthcare products Regulatory Agency has subsequently altered its position and it now permits claims for medical device products that kill lice by physical means, such as dehydration or asphyxiation, in line with other EU member states.
Outcomes
The primary outcome measure for both studies was elimination of lice following two applications of treatment. Checks for efficacy were performed on days 2, 6, 9 and 14 (± one day) using plastic detection combs on dry hair. At assessments, the hair was sectioned and systematically combed from scalp to tip. A separate comb was provided for each participant. If lice were found during assessments the insects were removed and taped to the case record. Anyone with lice 14 days after enrolment was supplied with phenothrin 0.5 per cent liquid.
Sample size
Trial A was structured partly to determine the sample size required for a larger second trial.We assumed a success rate for IPM/C of 90 per cent, based on in vitro studies and a clinical study conducted in Canada that incorporated combing following treatment,9 and estimated that efficacy for permethrin would be between 50-60 per cent. The sample size of 37 per group was considered to have at least 80 per cent power to detect (with 95 per cent confidence) a difference of 35 per cent between the success rates for IPM/C and permethrin. The high efficacy of IPM/C and the unexpectedly poor results for permethrin meant that the superiority of IPM/C was already supported by the results of trial A. Therefore, the larger trial B was designed with the main objective of obtaining fuller data on possible adverse events associated with IPM/C and to obtain efficacy data on a wider clinical population.
Randomisation
Treatments were randomised using a computer-generated list. The randomisation list for trial A was in balanced blocks of eight. In trial B, the randomisation was more complex due to the uneven distribution of treatment allocation. For this study, randomisation used blocks of 10, each of which was balanced in a 4:1 ratio between the two treatments. Investigators conducting enrolments were unaware of the randomisation block sizes.
Treatment allocation documentation was enclosed in sealed, sequentially numbered, opaque envelopes each allotted to its numbered case record book (CRB). Each investigator involved in treatment was issued with CRBs in batches and was unaware of the relationship of the numbers to the randomisation blocks. At enrolment participants were allocated the next available number in the sequence held by the investigator. Envelopes were only opened, and the allocation revealed, after participant details were entered into the CRB.
As randomisation was by individual, household members could receive different treatments, with the possibility that reinfestation risk could be increased for one treatment group if the other treatment was less successful. The difference between reinfestation after cure and treatment failure was determined by an algorithm that was believed to be sufficiently rigorous that it was unlikely treatment failures would be mistaken for reinfestation (Panel 2).
Panel 2: Treatment outcome definitions
Cure
- No lice present at day 9 or 14
Reinfestation after cure
- Adult lice and/or stage 3 nymphs must not be present at day 2 or 6
- Stage 1 or 2 nymphs must not be present at day 9 or 14
- If stage 3 nymphs are present at day 9 there must be no stage 1 or 2 nymphs present at day 6
- Stage 3 nymphs may be present at day 9 or 14 although there must not be more than two
- Adult lice should not exceed two at either day 9 or 14
Ovicidal failure
- Specific lice must be present at day 9 and 14 as below
- At day 9 there must be no adult lice and no stage 2 or 3 nymphs
- At day 14 there must be no adult lice or stage 3 nymphs
Treatment failure
- All other outcomes classed as treatment failure
Randomisation was not by cluster (ie, household) partly in order to assess different treatments within the same household and partly because the stratification process would have required numbers of participants considerably in excess of those required to demonstrate the main objectives of the studies.
Blinding
This investigation was divided between two randomised controlled trials. Both were assessor blinded. The physical forms of the products are sufficiently different that double blinding was impossible. As treatment was open, after the allocation had been revealed, investigators who applied treatment to a participant were not permitted to perform assessments on that person/family. Different investigators, blinded to the treatment, performed the post-treatment assessments. Participants were asked not to reveal their treatment to the assessors. Any lice collected during these examinations were fixed to the CRB using clear adhesive tape. The development stage or gender of the lice was subsequently determined by another investigator, also blind to treatment, using a microscope. This process enabled cases of reinfestation to be distinguished from cases of treatment failure.
Statistical methods and analysis
Groups were compared using Fisher’s exact test for presence/absence variables and the Mann-Whitney U-test for ranked variables. Differences in success rates between the treatments were quantified by the 95 per cent confidence interval, calculated using a normal approximation to the binomial distribution.
Additional analyses of success rate stratified for family (so that comparisons were made within family and then combined) and by trial gave conclusions that were essentially identical to those from the simpler unstratified analyses reported here, in each case showing a highly significant (P<0.001) advantage of IPM/C over permethrin.
Ethical approval
Ethical approval for trial A was granted by Cambridge Research Ethics Committee and for trial B by Hertfordshire 1 Research Ethics Committee.
Results
Participant flow
Over the two studies, we randomly assigned 111 participants to receive IPM/C and 57 to permethrin. Of these, four children (two from each group) were later excluded from the per-protocol analysis for trial A: three because it was later found that they had been treated with insecticide less than 14 days before enrolment and one who was given the incorrect second treatment. One participant in trial B (treated with IPM/C) had an incomplete follow up record, having missed the day 9 check-up. Each of these was included in the intention to treat analysis and conservatively treated as a treatment failure (Figure 1).
Recruitment
Both studies were conducted in Cambridgeshire, in 2005 and 2006. Trial A enrolled 60 children and 14 adults, and trial B enrolled 81 children and 13 adults.
Baseline data
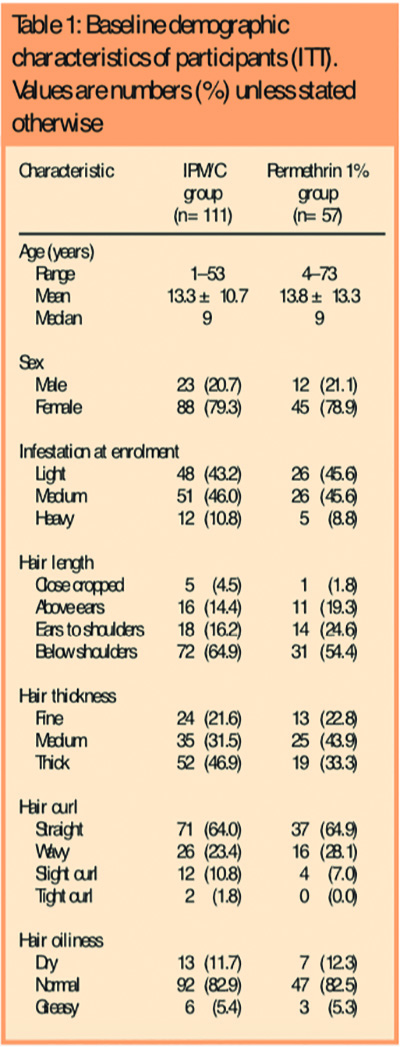
There were no statistically significant differences between the groups in respect of age, gender, intensity of infestation at baseline, hair length, thickness, degree of curl, or whether the hair was dry or greasy, although there was a trend in that those treated using IPM/C were more likely to have longer and thicker hair. The demographics of the groups at entry are shown in Table 1.
Outcomes and estimation
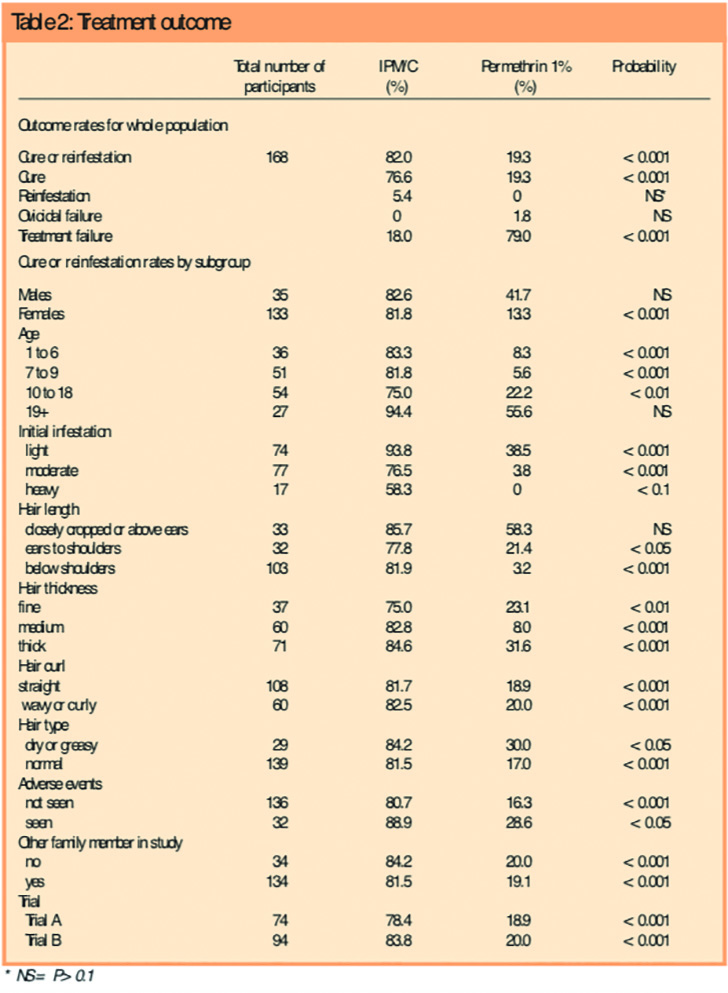
IPM/C had a much higher success rate than permethrin. For the combined data from the two trials, the intention to treat success rates were 82.0 per cent (91/111) for IPM/C, with 85 participants cured and six reinfested after cure, compared with 19.3 per cent (11/57,P<0.001) for permethrin, with 11 participants cured and 0 reinfested after cure. The difference in cure rate was 62.7 per cent (95 per cent confidence interval 50.2–75.2 per cent). Highly significant differences were found in favour of IPM/C in all subgroups analysed with the exception of subgroups where the participant numbers were small (Table 2). Following treatment with IPM/C, significantly fewer participants had live lice and the total number of lice recovered was significantly lower — around one-tenth — at each of the follow-up assessments compared with permethrin.
Ancillary analyses
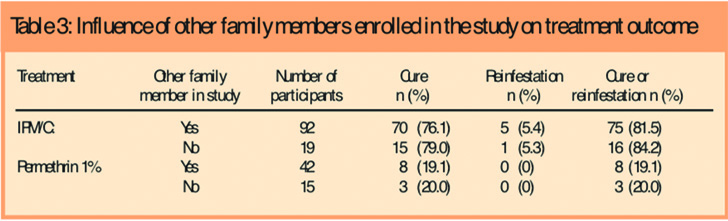
We considered the possibility that randomisation by individual, rather than by household, might have influenced outcome as different members of some households could be treated using materials with different efficacy, leading to a greater risk of reinfestation. Overall, we found no difference of outcome with either product, whether or not more than one member of the family was enrolled (Table 3).
Apart from five families (two all successes and three all failures, although the mix of treatments may have contributed to the outcome of the latter), there were six families where members received different treatments with mixed outcomes. In five of these, participants treated with permethrin were failures and those treated with IPM/C were successes. The sixth family included three participants; treatment of one with permethrin was successful, treatment of another with IPM/C was also successful and the treatment of the third, with IPM/C, failed. However, this last person was not available for the day 9 visit and was assumed to be a failure in the absence of data to confirm success.
For both treatments, the quantity of product applied depended on the length and thickness of hair but we found that over the two studies significantly more IPM/C (mean quantity 116.6g) was used than permethrin (mean 87.2g) (P<0.01). The increased amount of IPM/C used could have been related to the larger bottle size provided (more product was used in Trial B when a larger bottle was used) and the trend for longer, thicker hair in the IPM/C group. Additionally, IPM/C is applied to dry hair making it easier to see which parts of the hair require treatment. IPM/C is a mobile low surface tension fluid that often drips from longer hair so some could have been absorbed by towels used to protect the shoulders of the person being treated.
We found IPM/C significantly (P<0.001) easier to apply than permethrin. Participants found IPM/C to be significantly (P<0.001) more difficult to wash out and to leave the hair feeling greasy but said they were also significantly (P<0.001) more inclined to use the product again if they needed to treat a louse infestation.
Adverse events
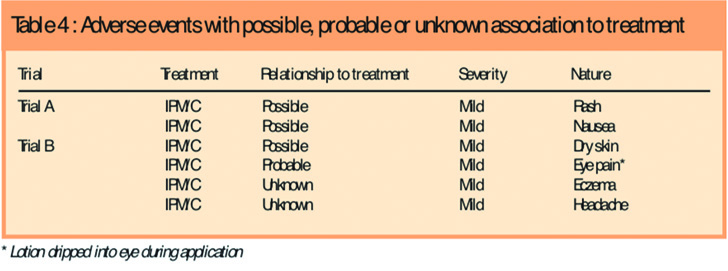
There was no significant difference between the treatments in the frequency, distribution or severity of adverse events, and no participant had treatment interrupted for an adverse event. There were 26 adverse events in 18 participants using IPM/C and 15 in 14 participants using permethrin (five participants had more than one event), representing an adverse event frequency of 16.2 per cent (18/111) for IPM/C and 24.6 per cent (14/57) for permethrin. Most adverse events were common childhood ailments. There was one serious adverse event (a broken arm), but this was unrelated to the product. All adverse events considered possibly or probably related, or with an unknown relationship to product were mild and occurred in the IPM/C group (Table 4).
Discussion
Interpretation
We have found that using two applications of IPM/C (Full Marks Solution) one week apart cured head louse infestation significantly more effectively than permethrin 1 per cent (Lyclear Creme Rinse). Cure (or reinfestation after cure) occurred in 91/111 participants (82.0 per cent) with IPM/C and 11/57 (19.3 per cent) with permethrin (P<0.001).
Generalisability
This study of the efficacy and safety of IPM/C followed closely the methodology used in previous trials conducted by us, and shows that IPM/C has a good cure (or reinfestation after cure) rate compared with other widely available head louse treatments in the UK.8 The outcome measure of no lice 14 days after first treatment is widely acknowledged as the benchmark for successful treatment.7 However, as evaluation of success by any method is not 100 per cent reliable with a single observation we have improved the sensitivity of our methodology by using two post-treatment assessments.
In the previously reported trial8 comparing dimeticone 4 per cent lotion with phenothrin 0.5 per cent liquid, little clear evidence of resistance to pyrethroids was identified, probably because that particular phenothrin product has a formulation designed to aid activity against louse eggs and is applied to dry hair and left on for 12 hours or overnight. However, the study described here showed that permethrin was considerably less effective, apparently due to higher than anticipated resistance, which would have a greater impact on a product applied to wet hair for only 10 minutes.
The fluid form of IPM/C resulted in investigators applying significantly greater quantities to most cases than they did to participants treated using permethrin creme rinse. Some of this was probably because IPM/C was applied to dry hair so the fluid was distributed throughout the hair mass in contrast to spreading the creme rinse over wetted hair. However, we did observe a greater use of IPM/C when the 100ml bottle was used compared with the 60ml bottle, indicating that an instruction to “thoroughly moisten the hair and scalp” is itself not only open to interpretation but can be influenced by the quantity of product available to the user. Thus a consumer who has purchased a 100ml, 200ml, or 300ml bottle of Full Marks Solution is likely to apply more product and, therefore, probably apply the product more efficiently, than a consumer using a 59ml bottle of Lyclear Creme Rinse who would be unlikely to purchase another bottle just in case one is insufficient. On this basis we anticipate that consumers using IPM/C may obtain better results simply because they are more likely to achieve a thorough coverage of the hair during treatment.
Unlike neurotoxic insecticides, the ingredients of Full Marks Solution do not cross the insect cuticle and have no pharmacological activity at the cellular level. From observations of applying the product directly to lice for 10 minutes in vitro, the mixture of isopropyl myristate (an oily fatty acid ester) and cyclomethicone (a low surface tension silicone fluid) appears to enter and block the tracheal breathing system of the louse, which rapidly immobilises the insect. The mixture also coats the surface of lice with a thin film of fluid that appears to dissolve into the lipid coat of the insect. The result is damage to the lipid coat (that may be enhanced by the process of washing the product out), which renders the louse more susceptible to water loss and so the louse becomes shrunken and dehydrated, and death follows. There are no known mechanisms by which head lice could develop a resistance to this mode of action.
Conclusion
The constituents of Full Marks Solution are widely used components of cosmetic preparations and do not present any toxicological hazard in repeated use. The short treatment time may be attractive to consumers, resulting in improved compliance. These factors, together with efficacy in use, suggest that the product is an acceptable preparation for the treatment of head louse infestation that overcomes current problems associated with insecticide resistance.
Acknowledgements
The studies were sponsored by SSL International Plc.
Competing interests
Ian Burgess has been a consultant to various makers of pharmaceutical products, alternative therapies, and combs for treating louse infestations. Peter Lee has analysed similar studies for other pharmaceutical companies. Christine Brown: none declared.
This paper was accepted for publication on 6 February 2008
About the authors
Ian F. Burgess, MPhil, FRES, is director and Christine M. Brown, RGN, SN Cert, is trial co-ordinator/lead investigator at the Medical Entomology Centre, Insect Research & Development Ltd, Royston. Peter N. Lee, MA, is statistician at P.N. Lee Statistics and Computing Ltd, Surrey.
Correspondence to: Ian Burgess, Medical Entomology Centre, Insect Research & Development Ltd, Cambridge House, Barrington Road, Shepreth, Royston SG8 6QZ (e-mail: ian@insectresearch.com).
References
- Thomas DR, McCarroll L, Roberts R, Karunaratne P, Roberts C, Casey D et al. Surveillance of insecticide resistance in head lice using biochemical and molecular methods. Archives of Disease in Childhood 2006;91:777–8.
- Willems S, Lapeere H, Haedens N, Pasteels I, Naeyaert J-M, De Maeseneer J. The importance of socio-economic status and individual characteristics on the prevalence of head lice in schoolchildren. European Journal of Dermatology 2005;15:387–92.
- Burgess IF, Brown CM, Peock S, Kaufman J. Head lice resistant to pyrethroid insecticides in Britain. BMJ 1995;311:752.
- Burgess IF, Brown CM. Management of insecticide resistance in head lice, Pediculus capitis (Anoplura: Pediculidae). In: Robinson WH, Rettich F, Rambo GW, editors. Proceedings of the 3rd International Conference on Urban Pests, Prague. Hronov: Grafické závody; 1999. p249–53.
- Hill N, Moor G, Cameron MM, Butlin A, Preston MS, Williamson MS et al. Single blind, randomised, comparative study of the Bug Buster kit and over-the-counter pediculicide treatments against head lice in the United Kingdom.BMJ 2005;331:384–6.
- Plastow L, Luthra M, Powell R, Wright J, Russel D, Marshall MN. Head lice infestation: bug busting vs. traditional treatment. Journal of Clinical Nursing 2001;10:775–83.
- Dodd CS. Interventions for treating head lice. Cochrane Database of Systematic Reviews 2001, Issue 2. Art. No.: CD001165. doi: 10.1002/14651858.CD001165. In: Cochrane Library, Issue 3, Oxford: Update Software; 2005.
- Burgess IF, Brown CM, Lee PN. Treatment of head louse infestation with 4% dimeticone lotion: randomised controlled equivalence trial. BMJ 2005; 330:1423–5.
- Kaul N, Palma KG, Maric A, de Rocquigny D, Silagy S, Lazer WJ. In vivo efficacy and safety of an experimental pediculicide rinse [poster]. 63rd Annual Meeting of the American Academy of Dermatology, New Orleans; 2005.


