See also (second paper) If a patient wants to take a statin, and offering it is in line with national guidance, which one should be recommended?
Abstract
Aim
This paper, the first of two, aims to provide an accessible overview of important recent publications on statins.
Design
Narrative review.
Content
The topic is reviewed under the following headings:
- Primary prevention: prescribing statins for people without established cardiovascular disease (CVD)
- Average risk reduction per 1.0mmol/L LDL cholesterol reduction
- Does taking a statin reduce the severity of myocardial infarction suffered despite the statin?
- Size of the absolute risk reduction achieved with statin depends on the baseline absolute risk as well as the LDL cholesterol reduction achieved
- The other element of the risk-benefit equation — adverse effects of statins
- Estimating a patient’s risk of a cardiovascular event — QRISK2
Conclusions
Recent meta-analyses on the use of statins by people without established cardiovascular disease (for primary prevention) show that their beneficial effect on all-cause mortality, if any, is small. For patients without established cardiovascular disease, diabetes or familial hypercholesterolaemia who are prescribed a statin, it would be reasonable for their clinician to re-estimate their CVD risk using QRISK2 and then discuss whether or not to continue the statin in view of the CVD risk estimate, the results of the meta-analyses and the evidence on adverse effects. Whether a patient chooses to continue the statin may depend, in part, on his or her attitude to risk and tablet taking.
Several papers add to the clinical guideline on lipid modification issued by the National Institute for Health and Clinical Excellence (NICE) in May 2008. They should influence choices within the framework set by NICE’s guidance and this review will summarise them. The content of this review should be viewed in the context of what is known about how people take medicines and the input people would like into decisions about their medicines, for example:
- Between a third and a half of medicines prescribed for long-term conditions are not used as recommended1
- Non-adherence is sometimes intentional and sometimes unintentional
- Most people want some input into decisions about their medicines: 39 per cent wanted the GP to share the decision, 28 per cent wanted the GP to be the main decision maker, 17 per cent wanted the GP to be the only decision maker and 14 per cent wanted the patient to be the main decision maker2
Recommendations in NICE’s guideline on medicines adherence3 include:
- Establishing what level of involvement in decision-making the patient would like
- Offering patients clear relevant information before prescribing; that information will probably include, but not be limited to, likely benefits and likely adverse effects
- Being aware that greater patient involvement may mean the patient decides not to take or to stop taking a medicine; if in the healthcare professional’s view this could have an adverse effect, the information provided to the patient on risks and benefits and the patient’s decision should be recorded
NICE’s guideline on lipid modification also states that people should be offered information about their absolute risk of cardiovascular disease and about the absolute benefits and harms of an intervention over a 10-year period.4 Some of the figures below will highlight the relevance of this.
The net ingredient cost of statins dispensed in the community in England in 2009 was £460m, representing 5.4 per cent of the total for prescription items. It is appropriate to focus on using statins as sensibly as possible.
Primary prevention
Prescribing statins for people without established cardiovascular disease
Meta-analyses on statins for primary prevention were published in the BMJ in 20095 and Archives of Internal Medicine in June 2010.6
The BMJ meta-analysis reported odds ratios, relative risk reductions and absolute risks of events. The latter can be converted into “numbers needed to treat” to avoid one event.
If a treatment reduces the risk of an event from 1 per cent to 0.5 per cent, the relative risk reduction is 50 per cent, the absolute risk reduction is 0.5 per cent and the number of people who need to be treated to avoid one event (NNT) is 200. The mean follow-up in the trials included in the analysis was 4.1 years (see Panel 1).
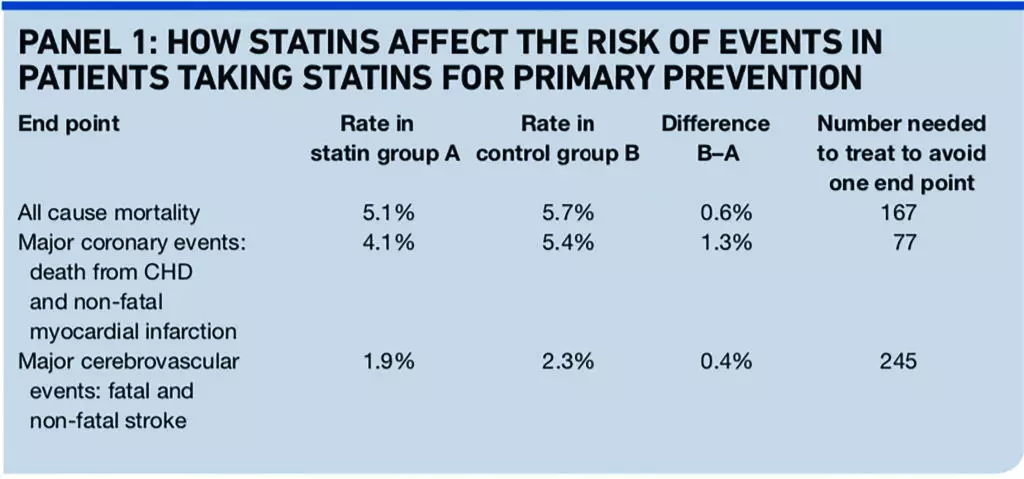
The event rates in the control group indicate that the trial patients’ average risk of cardiovascular disease was probably a little lower than the conventional threshold for offering a statin (20 per cent over 10 years).
So the numbers needed to treat will be a slight underestimate of the likely effect in people offered a statin in line with NICE’s guidance, many of whom have cardiovascular risks substantially above 20 per cent over 10 years.
Unfortunately, about 6 per cent of patients in the meta-analysis had a previous cardiovascular event (treatment with statin was secondary prevention rather than primary prevention). The authors report that excluding the three trials that enrolled these patients did not influence the outcome of the analysis, but the more recent Archives of Internal Medicine meta-analysis is “cleaner”, based on data from only individuals without clinically manifest cardiovascular disease.6
The Archives of Internal Medicine meta-analysis used data from 65,229 trial participants followed for approximately 244,000 person-years, during which 2,793 died. The average treatment period was 3.7 years. Use of statins was not associated with a statistically significant reduction in the risk of all-cause mortality (risk ratio 0.91; 95 per cent confidence interval 0.83-1.01).
Setting aside, for a moment, the lack of statistical significance within the combined dataset with a mean placebo mortality rate of 11.4 per 1,000 person years, there were on average an estimated seven fewer deaths for every 10,000 person-years of treatment. The absolute risks of death were a rate of 4.1 per cent in the statin group and 4.4 per cent in the control group, a difference of 0.3 percentage points, which was not statistically significant.
This suggests that in the short term, for true primary prevention (people without established cardiovascular disease) the reduction of all-cause mortality, if any, is small, while the reduction in non-fatal major cardiovascular events is important. Some would argue that cardiovascular mortality, rather than all-cause mortality, would be a more appropriate outcome to use.
An editorial accompanying the second meta-analysis points out that the trials are short in comparison with clinical use that may go on for decades.7 Advocates of statins for primary prevention assert that cumulative benefit will accrue over a longer time, while sceptics postulate that low-hanging fruit is picked early and little incremental benefit accrues later.
The data fail to provide conclusive support either to the increasing returns or diminishing returns beliefs. While sincere advocates on both sides will try to convince us otherwise, the truth is that we do not know.7 If compliance is better in clinical trials than in patients not participating in a trial, this will tend to make the number of patients needed to treat in general practice higher than those found in trials.
The JUPITER trial
The JUPITER trial potentially provides a strong argument for using rosuvastatin 20mg daily for primary prevention. A critical reappraisal has found the trial to be flawed, however, concluding that its results do not support the use of statin treatment for primary prevention of cardiovascular disease.8
JUPITER found — in primary prevention — a reduction in coronary heart disease complications worthy of attention.9 Some 17,802 apparently healthy people with low-density lipoprotein (LDL) cholesterol levels of <3.4mmol/L and high-sensitivity C-reactive protein levels of ≥2.0mg/L were randomised to rosuvastatin 20mg daily (a fairly high dose) or placebo. The combined primary endpoint was myocardial infarction, stroke, arterial revascularisation, admission to hospital for unstable angina, or death from cardiovascular causes. The trial was stopped after a median follow-up of 1.9 years after a highly statistically significant improvement in the primary end point with rosuvastatin (hazard ratio 0.56; 95 per cent confidence interval 0.46–0.69; P<0.00001).
The reappraisal that concluded that JUPITER was flawed found, for example, that cardiovascular mortality in the trial was surprisingly low compared with total mortality and that of those people who did suffer a myocardial infarction, far fewer died than would have been expected.8 These findings seem compelling. No response to them by the authors of the paper in question has been published in the nine issues of Archives of Internal Medicine published since (up to and including the 22 November 2010 issue). Limitations of the JUPITER data set will impact on secondary analyses of subgroups, a number of which have been published, and on meta-analyses which include the data, including both of those cited earlier.
An editorial that comments on the reappraisal notes that there is increasing scepticism about the early stopping of randomised trials, especially when the number of outcomes is small.7 Early termination for benefit tends to introduce bias, exaggerating benefit and underestimating harm.
Average risk reduction per 1.0mmol/L LDL cholesterol reduction
The Cholesterol Treatment Trialists’ (CTT) Collaboration recently reported a meta-analysis on individual patient data from 170,000 individuals in randomised trials of statin therapy versus control (21 trials) or more versus less intensive statin regimens (five trials). Each 1.0mmol/L reduction in LDL cholesterol at one year after randomisation reduced the annual rate of major vascular events by just over a fifth. There was no evidence of any threshold within the cholesterol range studied, suggesting that reduction of LDL cholesterol by 2–3mmol/L would reduce risk by about 40–50 per cent.10
These data facilitate another approach to discussing the size of potential benefit of taking a statin with individual patients. When statin treatment for primary prevention is being considered and a cardiovascular risk estimation tool has been used to assess the patient’s baseline risk of a cardiovascular event, the relative risk reduction from the CTT Collaboration meta-analysis can be applied to this baseline risk to provide the patient with a more personal (and accurate) estimation of their potential benefits.
Does taking a statin reduce the severity of myocardial infarction suffered despite the statin?
A retrospective analysis of a Swedish disease register, which provides less strong evidence than a randomised controlled trial, included 103,459 consecutive patients admitted with a first acute myocardial infarction.11
It found that use of aspirin, beta-blockers, ACE inhibitors, or statins before hospital admission for a first myocardial infarction was associated with less risk of presenting with ST-segment elevation, a marker of larger infarctions. The risk of presenting with an ST-segment elevation MI (STEMI) was lower in patients who had used two or more of these drugs (see Panel 2).
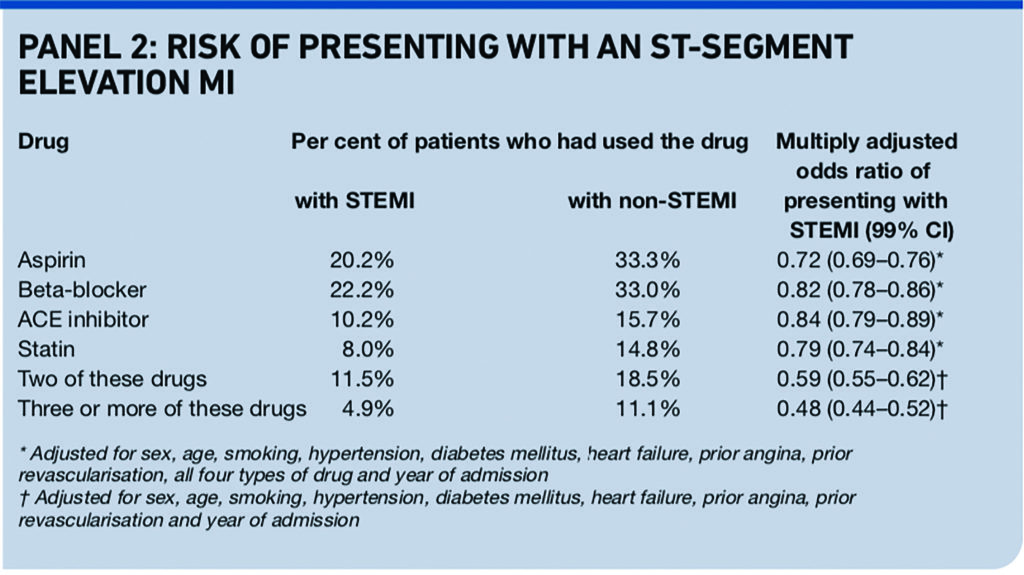
Results from an earlier analysis of a different registry of patients presenting with acute coronary syndrome (not limited to first myocardial infarction) and their use of statins are consistent with the Swedish analysis.12
Size of the absolute risk reduction achieved with statin depends on the baseline absolute risk as well as the LDL cholesterol reduction achieved
People who have clinically manifest cardiovascular disease are at high risk of a cardiovascular event, such as myocardial infarction or stroke. As an indication, in the 4S trial, in which patients with angina pectoris or previous myocardial infarction were randomised to simvastatin or placebo with a median follow-up period of 5.4 years, 28 per cent of patients in the placebo group had a major coronary event (coronary death, definite or probable hospital-verified non-fatal acute myocardial infarction, resuscitated cardiac arrest, or definite silent myocardial infarction verified by ECG).13
This suggests that the patients in 4S were at higher baseline risk than the conventional threshold risk for offering statin for primary prevention (20 per cent or greater 10-year risk of developing cardiovascular disease). The relative risk reduction per 1.0mmol/L LDL cholesterol reduction is similar irrespective of baseline absolute risk of a cardiovascular event,10 so patients with the highest baseline absolute risk obtain the largest reduction in absolute risk. In 4S the number needed to treat to avoid one death was 30 and to avoid one major coronary event was 15.
The other element of the risk-benefit equation — adverse effects of statins
Also relevant when deciding whether to prescribe or take a statin will be the risk and nature of adverse effects. Four recent publications add to the picture:
First, the CTT Collaboration meta-analysis of individual data from randomised trials found no significant effects on deaths due to cancer or other non-vascular causes or on cancer incidence.10 Cases of rhabdomyolysis were also sought. An excess of four (standard error 2) per 10,000 was found in the five trials of more versus less intensive statin therapy, compared with one (standard error 1) per 10,000 in the trials of standard statin regimens versus control. All of the excess with more intensive therapy occurred in the two trials of simvastatin 80mg versus 20mg daily.
The BMJ meta-analysis on statins for primary prevention also found no evidence of increased risk of cancer.5
Second, a population cohort study using the QResearch database quantified a number of adverse effects that have been found to be associated with statin use.14 The study was not designed to show causality but found the associations with statin use shown in Panel 3.
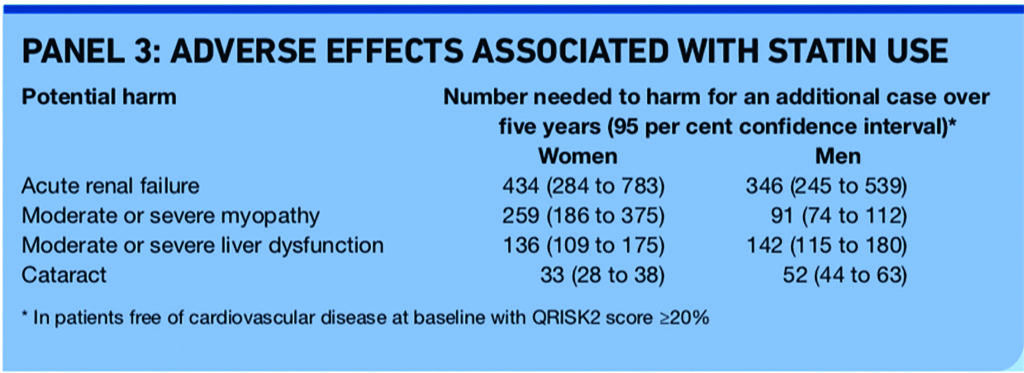
Hazard ratios used to calculate the number needed to harm (NNH) were adjusted for potential confounding variables, although the possibility of residual bias or confounding cannot be discounted. For example, the hazard ratios for acute renal failure were adjusted for variables including age, treated hypertension (including use of ACE inhibitors which can cause acute renal failure), smoking, diabetes, heart failure and stage 3b+ kidney disease, but not for non-steroidal anti-inflammatory drug use.14 The association of statin use with acute renal failure was supported by evidence suggesting a dose-response effect, by the risk returning to normal one to three years after stopping statin and by self-controlled case series analysis.14
An accompanying editorial states that this is the best available “real world” estimate of the risk of adverse events with statin treatment.15
Third, the Medicines and Healthcare products Regulatory Agency (MHRA) has reported the outcome of a Europe-wide review of statins.16 The headline message was that the balance of risks and benefits of statins as a class remains positive. Statins are generally well tolerated by most people who use them. However, the review concluded that it was important that prescribers and patients are aware of the potential for adverse reactions it identified:
- Treatment with any statin may be associated with depression, sleep disturbances, memory loss and sexual dysfunction.
- Statins may very rarely be associated with interstitial lung disease. Patients should seek help from their doctor if they develop presenting features of interstitial lung disease such as dyspnoea, non-productive cough, and deterioration in general health, such as fatigue, weight loss or fever
Fourth, the simvastatin product information has been updated to include warnings about increased risk of myopathy in patients receiving the highest licensed dose (80mg/day).17 The update follows a review of the SEARCH trial. This evaluated the effect of simvastatin 80mg versus 20mg on major vascular events in 12,064 patients with a history of myocardial infarction over a median follow-up of 6.7 years.
Taking 80mg/day did not provide any significant benefits over 20mg/day. However myopathy occurred in 0.9 per cent of those assigned to 80mg compared with 0.02 per cent assigned 20mg. An estimated 11 patients in the 80mg group developed rhabdomyolysis compared with none in the 20mg group. The MHRA’s advice includes:
- Simvastatin 80mg should be considered only in patients with severe hypercholesterolaemia and high risk of cardiovascular complications who have not achieved their treatment goals on lower doses, when the benefits are expected to outweigh the potential risks.
- Prescribers treating patients who are taking simvastatin 80mg or who are being considered for an up-titration to that dose may need to review their treatment during the next visit, to take into account the new evidence.
- Patients taking simvastatin 80mg should not stop. However, they should be advised to contact their doctor immediately if they experience unexplained muscle pain, tenderness or weakness.
The National Prescribing Centre has added some context.18 Statin-related myopathy occurs with all statins and the risk increases at higher doses. There is no good evidence to suggest that any one statin has any advantages over another in this regard at a population level when used at equivalent doses. Several trials that compared atorvastatin 80mg/day with atorvastatin or other statins at lower doses found that atorvastatin 80mg/day was associated with more discontinuations due to adverse effects than the comparator statin. The MHRA has previously warned about the risk of muscle side effects with rosuvastatin 40mg.
Estimating a patient’s risk of a cardiovascular event — QRISK2
A key feature of the NICE guidance on lipid modification for primary prevention is that a full formal risk assessment, including use of a cardiovascular disease risk estimation tool, should precede any use of a statin for primary prevention. The guidance states that cardiovascular disease risk estimation tools can provide only an approximation of cardiovascular disease risk. It says: “Interpretation of cardiovascular disease risk scores should always reflect informed clinical judgment.”
NICE has ceased to recommend any specific cardiovascular disease risk estimation equation, leaving prescribers to choose the equations and tool they consider to be most appropriate. So is the more recent QRISK2 tool better than the modified Framingham risk equation that NICE used to recommend?
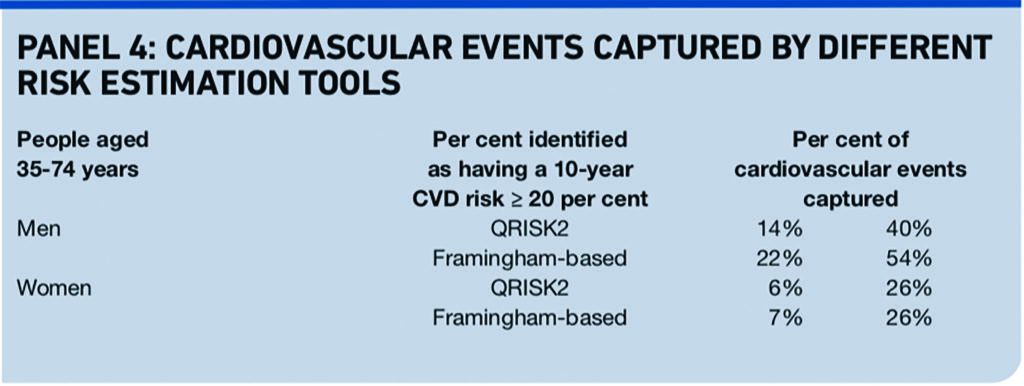
The answer, from an independent and external validation published recently, is “probably yes”.19,20 Neither QRISK2 nor the modified Framingham equation is great at identifying everyone who goes on to have a cardiovascular event, but QRISK2 is better at differentiating between those who go on to have an event and those who do not and more accurately estimates an individual’s risk.
QRISK2 reclassifies 43 per cent of women and 45.4 per cent of men labelled high risk (≥20% at 10 years) by the modified Framingham equation into a low risk group, <20 per cent. The correlation of predicted risk with observed risk at all levels of risk was much stronger for QRISK2 than for the modified Framingham equation formerly recommended by NICE.
Against this, QRISK2 identifies fewer men who go on to have a cardiovascular event than does the modified Framingham equation.19
QRISK2 can be used on the internet at www.qrisk.org. Whichever risk estimation tool is used, it is important to be fully aware of the correct way to use it, adjusting appropriately for relevant variables that fall outwith the tool.
Conclusions
We have seen that recent meta-analyses on the use of statins by people without established cardiovascular disease (for primary prevention) show that their beneficial effect on all-cause mortality, if any, is small. If treatment decisions are informed by use of a cardiovascular disease risk estimation tool, as NICE recommends, the more accurate risk estimation provided by QRISK2 seems a good thing.
For patients without established cardiovascular disease, diabetes or familial hypercholesterolaemia who are prescribed a statin for primary prevention it would be reasonable to:
- Re-estimate their cardiovascular disease risk using QRISK2 and their pre-treatment levels of total cholesterol and HDL cholesterol. If these pre-treatment values are not available it is generally safest to assume that cardiovascular disease risk is higher than that predicted by current levels of lipids on treatment. If the patient is taking antihypertensive medicines his or her current blood pressure on treatment can be used with QRISK2.
- Discuss with them whether or not to continue the statin in view of (a) the cardiovascular disease risk estimate, (b) the results of the meta-analyses, by using the relative risk reduction per 1.0mmol/L LDL cholesterol reduction or the numbers needed to treat, and (c) the evidence on the risk of adverse effects. Whether or not a patient chooses to continue or stop the statin may depend, in part at least, on his or her attitude to risk and tablet taking.
Acknowledgements
I thank the GPs, hospital consultants and pharmacists who read and commented on drafts of this paper.
About the author
Chris Corfield is chief pharmacist at NHS Hammersmith and Fulham (email Christopher.Corfield@hf-pct.nhs.uk)
References
1. Horne R, Weinman J, Barber N, Elliott R, Morgan M. Concordance, adherence and compliance in medicine-taking. Report for the National Co-ordinating Centre for NHS Service Delivery and Organisation R&D 2005.
2. Cox K, Britten N, Hooper R, White P. Patients’ involvement in decisions about medicines: GPs’ perceptions of their preferences. British Journal of General Practice 2007;57:777–84.
3. NICE clinical guideline 76. Medicines adherence — involving patients in decisions about prescribed medicines and supporting adherence. London: NICE, 2009.
4. NICE clinical guideline 67. Lipid modification — cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease. London: NICE, 2008.
5. Brugts J, Yetgin T, Hoeks S, Gotto A, Shepherd J, Westendorp R et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ 2009;338:b2376.
6. Ray K, Seshasai S, Erqou S, Sever P, Jukema J, Ford I et al. Statins and all-cause mortality in high-risk primary prevention. A meta-analysis of 11 randomized controlled trials involving 65229 participants. Archives of Internal Medicine 2010;170:1024–31.
7. Green L. Cholesterol-lowering therapy for primary prevention. Archives of Internal Medicine 2010;170:1007–8.
8. de Lorgeril M, Salen P, Abramson J, Dodin S, Hamazaki T, Kostucki W et al. Cholesterol lowering, cardiovascular disease, and the rosuvastatin-JUPITER controversy. A critical reappraisal. Archives of Internal Medicine 2010;170:1032–6.
9. Ridker P, Danielson E, Fonseca F, Genest J, Gotto A, Kastelein J et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. New England Journal of Medicine 2008;359:2195–207.
10. Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010;376:1670–81.
11. Björck L, Wallentin L, Stenestrand U, Lappas G, Rosengren A. Medication in relation to ST-segment elevation myocardial infarction in patients with a first myocardial infarction. Archives of Internal Medicine 2010;170:1375–81.
12. Spencer F, Allegrone J, Goldberg R, Gore J, Fox K, Granger C et al. Association of statin therapy with outcomes of acute coronary syndromes: the GRACE study. Annals of Internal Medicine 2004;140:857–66.
13. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4,444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383–9.
14. Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ 2010;340:c2197.
15. Alsheikh-Ali A. Balancing the intended and unintended effects of statins. BMJ 2010;340:c2240.
16. Medicines and Healthcare products Regulatory Agency. Statins: updated product information in patient leaflets on adverse reactions. Drug Safety Update 2009;3(4):11.
17. Medicines and Healthcare products Regulatory Agency. Simvastatin: increased risk of myopathy at high dose (80mg). Drug Safety Update 2010;3(10):7.
18. National Prescribing Centre. What is the place in therapy of simvastatin 80mg in the light of recent MHRA guidance? MeReC Monthly 2010;(July):1–2.
19. Collins G, Altman D. An independent and external validation of QRISK2 cardiovascular disease risk score: a prospective open cohort study. BMJ 2010;340:c2442.
20. Scott I. Improving the accuracy of predicting cardiovascular risk — QRISK2 supersedes Framingham as the risk prediction score of first choice. BMJ 2010;340:c2334.