Key points:
- Avibactam is a novel non-β-lactam β-lactamase inhibitor;
- By inhibiting many serine β-lactamases, avibactam restores the activity of ceftazidime against many Enterobacteriaceae;
- Limited data from phase II and III clinical trials support its use for patients with complicated intra-abdominal infections and complicated urinary tract infections with insufficient treatment options;
- Use should be reserved for patients with limited to no options for the treatment of multidrug-resistant Gram-negatives;
- Further studies are warranted to assess its clinical efficacy for treatment of bacteraemia and nosocomial pneumonia owing to carbapenemase-producing Enterobacteriaceae or multidrug-resistant Pseudomonas aeruginosa.
Introduction
Developing new antimicrobial agents is one of the seven key areas for action in the UK five-year antimicrobial resistance strategy[1]
. Ceftazidime-avibactam is a new cephalosporin and β-lactamase inhibitor combination antibiotic that received Food and Drug Administration (FDA) approval in February 2015 and marketing authorisation by the European Medicines Agency (EMA) in April 2016[2]
. It is one of seven new antimicrobials since the introduction of the Infectious Diseases Society of America’s call to action with the “10 x ’20” initiative to improve the antimicrobial pipeline[3],
[4]
. This new antimicrobial has activity against some multidrug-resistant isolates including carbapenem-resistant Enterobacteriaceae (CRE), which were categorised as “an urgent threat”, and extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae, categorised as a “serious threat”, by the Centers for Disease Control and Prevention[5]
. Ceftazidime-avibactam was designated a Qualified Infectious Diseases product eligible for fast-track review. Given the potential to respond to an unmet medical need, the FDA approved the antibiotic combination in February 2015 based on pharmacokinetic/pharmacodynamic analyses, phase II clinical data, and the prior efficacy and safety data of ceftazidime monotherapy[2]
. This article is a review of the published data on ceftazidime-avibactam including the mechanism of action and resistance, spectrum of activity, pharmacokinetics and dynamics, and clinical efficacy and safety.
Chemistry, structure and function
Ceftazidime is a third-generation, semisynthetic cephalosporin. It is a pentahydrate of (6R, 7R,Z)-7-(2-(2-aminothiazol-4-yl)-2-(2-carboxypropan-2-yloxyimino)acetamido)-8-oxo-3-(pyrindium-1-ylmethyl)-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylate (see Figure 1)[2],
[6]
. The 2-aminothiazole group at the R1 side chain improves the binding affinity for penicillin-binding protein (PBP)-3 of Gram-negative organisms[7],
[8]
. In addition, the α-carbon dimethylacetic acid group and propylcarboxy side chain groups improve the potency against Pseudomonas aeruginosa
[7],
[8]
.
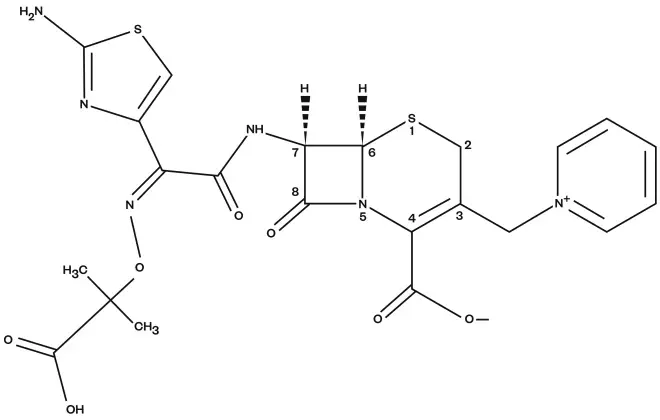
Figure 1: Chemical structure of ceftazidime
Ceftazidime is a third-generation, semisynthetic cephalosporin
Avibactam is the first non-β-lactam β-lactamase inhibitor (BLI). The structure is a diaza-bicyclo octane core instead of the β-lactam core used by tazobactam and sulbactam (see Figure 2)[2],
[6]
.
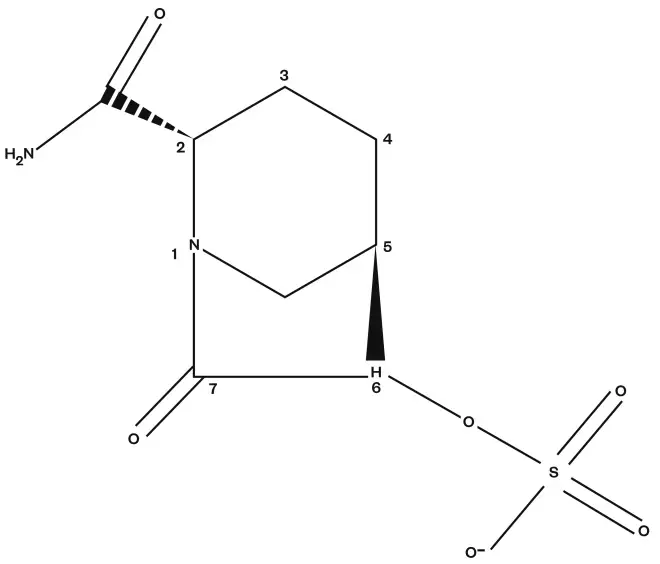
Figure 2: Chemical structure of avibactam
Avibactam is the first non-β-lactam β-lactamase inhibitor (BLI). The structure is a diaza-bicyclo octane core instead of the β-lactam core used by tazobactam and sulbactam
Mechanism of action
The mechanism of action of ceftazidime is well known and, like other cephalosporins, it inhibits bacterial cell wall synthesis. Ceftazidime binds to and inhibits penicillin-binding proteins that prevent the final transpeptidation step of peptidoglycan synthesis in the bacterial cell wall[2]
. Avibactam inhibits many serine β-lactamases by covalently binding and inhibiting the enzymes. Unlike the currently available β-lactam BLIs, including tazobactam, the bond is slowly reversible via deacylation, allowing some recycling of avibactam[9],
[10]
.
Susceptibility testing
Antimicrobial susceptibility testing for ceftazidime-avibactam can be performed by either microbroth dilution or disk diffusion[2]
. The Clinical Laboratory Standards Institute (CLSI) susceptibility breakpoint for ceftazidime monotherapy is set at 4mg/L or lower for Enterobacteriaceae and 8mg/L or lower for P. aeruginosa
[11]
. However, neither CLSI nor the European Committee on Antimicrobial Susceptibility Testing (EUCAST) currently have breakpoints set for ceftazidime-avibactam[11],
[12]
. The only interpretive criteria are from the FDA for Enterobacteriaceae and P. aeruginosa (see Table 1)[2]
. The FDA recommends minimum inhibitory concentration (MIC) values are determined with a fixed concentration of 4μg/ml of avibactam[2]
.
| Minimum inhibitory concentration (mg/L) | Zone diameter (mm) | |||
|---|---|---|---|---|
| Organism | Susceptible | Resistant | Susceptible | Resistant |
| Enterobacteriaceae | ≤ 8/4 | ≥ 16/4 | ≥ 21 | ≤ 20 |
| Pseudomonas aeruginosa | ≤ 8/4 | ≥ 16/4 | ≥ 18 | ≤ 17 |
Antimicrobial activity
Avibactam can inhibit Ambler class A (e.g. TEM, SHV, and CTX-M), C (ex. AmpC) and some class D (e.g. OXA-48) β-lactamases[8]
,[13]
,[14]
,[15]
,[16]
,[17]
. By binding to and inhibiting enzymes in these classes, the Gram-negative activity of ceftazidime is restored (see Table 2).
Enterobacteriaceae
Ceftazidime-avibactam is active against a wide variety of Enterobacteriaceae including Escherichia coli, Klebsiella pneumoniae and Enterobacter spp.[13]
,[14]
,[15]
,[16]
,[17]
. With the addition of avibactam, most large in vitro studies have demonstrated MIC90 concentrations significantly lower than ceftazidime monotherapy and below the FDA breakpoint for ceftazidime-avibactam[13]
,[14]
,[15]
,[16]
,[17]
. For E. coli and K. pneumoniae isolates producing class A extended spectrum β-lactamases including TEM, SHV and CTX-M, avibactam signficantly reduces the MIC90 for ceftazidime; for some isolates over 256-fold[13],
[14]
. Avibactam restores ceftazidime activity against Citrobacter and Enterobacter spp. producing chromosomal class C cephalosporinases[13]
. Against a collection of 42 K. pneumoniae isolates producing KPC carbapenemases, avibactam was effective at restoring activity to ceftazidime for all strains[15]
. In addition, ceftazidime-avibactam was active in vitro against Enterobacteriaceae isolates producing class D OXA-48 carbapenemases[16],
[18]
.
Non-lactose fermenters
Ceftazidime-avibactam has variable activity against non-lactose fermenters including P. aeruginosa and Acinetobacter baumannii
[19]
. Avibactam restored ceftazidime activity for P. aeruginosa strains with derepressed AmpC enzyme production[19]
. However, ceftazidime-avibactam only had limited activity against strains with OXA-related ESBLs or up-regulated efflux pumps[19]
. In a global survelliance study of urinary tract isolates, ceftazidime-avibactam was only active in vitro against around 50% of P. aeruginosa isolates that were nonsusceptible to ceftazidime or meropenem[20]
. Ceftazidime-avibactam did not have better in vitro activity than ceftazidime monotherapy for a collection of 51 carbapenem-resistant A. baumannii producing a variety of different OXA β-lactamases[19]
.
Against a collection of 54 Burkholderia
cepacia complex isolates from patients with cystic fibrosis, the addition of avibactam improved the activity of ceftazidime from 9% susceptible to 67% susceptible[19]
.
Anaerobes
Ceftazidime-avibactam has limited activity against anaerobic bacteria[21]
. Compared to ceftazidime monotherapy, the MIC90 for Bacteroides fragilis was significantly reduced (>128 to 16)[21]
. However, for other Bacteroides
species, Fusobacterium species and other anaerobes, the activity of ceftazidime-avibactam was limited[21]
. Combination therapy with metronidazole generally found additive or indifferent effects[21]
.
Gram-positive organisms
Similar to ceftazidime monotherapy, ceftazidime-avibactam has limited activity against Gram-positive isolates. The MIC90 for β-haemolytic streptococci was 0.5mg/L but was significantly higher for Staphylococcus aureus (MIC90 >32mg/L)[20]
.
| Ceftazidime | Ceftazidime-avibactam | |||
|---|---|---|---|---|
| MIC50 | MIC90 | MIC50 | MIC90 | |
| Enterobacteriaceae | ||||
| Citrobacter freundii | 0.25–16 | 128 | 0.125–0.25 | 0.5 |
| Enterobacter spp. | 0.5–128 | >128 | 0.125–1 | 1–8 |
| Escherichia coli | 0.25–16 | 32–>128 | 0.06–0.25 | 0.125–1 |
| ESBL-producing | 16–32 | 32–>32 | 0.008–0.125 | 0.008–0.25 |
| AmpC-hyperproducing | 16–32 | 32–>128 | 0.125 | 0.5 |
| Klebsiella pneumoniae | 0.5–128 | >128 | 0.125–1 | 0.5–4 |
| ESBL-producing | 8–32 | 32–>128 | 0.006–0.5 | 0.25–2 |
| OXA-48-producing | 256 | 512 | 0.25 | 0.5 |
| KPC-producing | 256–>512 | 256–>512 | 0.25 | 1–4 |
| Morganella morganii | 0.125–1 | 8–64 | 0.06–0.125 | 0.12–0.25 |
| Proteus mirabilis | 0.06–0.25 | 0.25–2 | ≤0.06 | 0.06–0.125 |
| Proteus vulgaris | 0.06–0.125 | 0.12–8 | ≤0.06 | 0.06–0.25 |
| Serratia marcescens | ≤0.25 | 1 | 0.25 | 0.5 |
| Non-lactose fermenters | ||||
| Acinetobacter baumannii | 8–>32 | >32 | 8–32 | 16–>32 |
| Carbapenem-resistant | 32–128 | 32–>512 | 32 | 32–256 |
| Pseudomonas aeruginosa | 2–4 | 32–64 | 2 | 4–32 |
| AmpC-derepressed | 64 | >128 | 4 | 8 |
| multidrug-resistant | >16 | >16 | 8 | 16–32 |
Resistance
Avibactam does not inactivate Ambler class B metallo-β-lactamases or many of the OXA enzymes (e.g. OXA-23, -40, -51, and -58) so organisms producing these types of enzymes will be resistant to ceftazidime-avibactam[19],
[26]
. As stated above, some multidrug-resistant P. aeruginosa isolates demonstrate resistance to ceftazidime-avibactam. In one investigation of ten different MDR isolates, the mechanisms most responsible for ceftazidime-avibactam resistance were discovered to be diminished outer membrane permeability and/or overexpression of efflux pumps[27]
. The first case report of ceftazidime-avibactam resistant KPC-3-expressing K. pneumoniae isolates was recently reported in the literature but the exact mechanism of resistance has yet to be elucidated[28]
. The frequency of spontaneous resistance in experimental models with P. aeruginosa and Enterobacteriaceae were low and related to point mutations at avibactam’s binding site[29],
[30]
.
Pharmacokinetics/pharmacodynamics
The pharmacokinetics of ceftazidime are well known and will not be discussed in detail here[31],
[32]
. The pharmacokinetics of avibactam were assessed in phase I clinical trials[33],
[34]
. In healthy adults, avibactam plasma concentrations are directly proportional to the increases in dose. Avibactam has a volume of distribution of 20 to 24L, a half-life of 1.5 to 2.7 hours, ~8% protein binding, and a renal clearance of 10.4 to 13.8L/hr[33]
[34]
,[35]
,[36]
,[37]
,[38]
. It is predominately renally excreted as unchanged drug. The clearance of avibactam is decreased with decreasing renal function; therefore, dosage adjustments are warranted for patients with renal impairment and dosages should be administered after haemodialysis on dialysis days[2]
. Table 3 summarises the pharmacokinetics of ceftazidime and avibactam at steady state following a two-hour infusion. The pharmacokinetics of both ceftazidime and avibactam are similar and simultaneous administration of both agents does not impact their individual pharmacokinetic profiles.
| Parameter | Vd(L) | Cmax (mg/L) | t ½, (hrs) | AUC (mg *hr/L) | % protein binding | % urinary elimination | % ELF penetration |
|---|---|---|---|---|---|---|---|
| Ceftazidime 2g every 8 hours | 17 | 90 | 2.7 | 291 | 21% | 83% | 21% |
| Avibactam 500mg every 8 hours | 22.2 | 14.6 | 2.7 | 38.2 | 8% | 97% | 25%–35% |
Similar to other cephalosporins, the best predictor of efficacy and antimicrobial activity of ceftazidime is the percentage of time that plasma concentrations of free (unbound) drug exceeds the MIC (f T > MIC). For cephalosporins, f T > MIC of ≥50% has been associated with clinical and microbiological efficacy[2],
[6],
[36],
[38]
. Avibactam alone lacks antimicrobial activity and thus MICs cannot be used to determine acceptable concentrations[6],
[36],
[38]
. However, it has been proposed that the percentage of time of the dosing interval that free BLI concentrations are above the threshold concentration (f T > CT) is the pharmacodynamic target for avibactam[6],
[36],
[38]
. CT is the concentration of BLI that must be maintained in combination with a β-lactam to inhibit bacterial growth[36],
[38]
. Data from neutropenic mouse lung and thigh P. aeruginosa infection models suggest avibactam efficacy f T > CT of ≥20%–40%; CT = 1µg/mL[40]
. When avibactam is combined with ceftazidime, the pharmacodynamics of the combination take on the same properties of the β-lactam when the concentration of the BLI is at, or above, the CT[36],
[38]
. From this data, it was determined that ceftazidime 2g-avibactam 0.5g infused over two hours every eight hours would have ≥98% probability of target attainment for MICs up to 8mg/L[36],
[38]
. This value decreases to approximately 50% and 1% for MICs of 16mg/L and 32mg/L respectively[36],
[38]
.
Clinical efficacy trials
Two phase II clinical trials were conducted to assess the safety and efficacy of ceftazidime-avibactam in the treatment of infections caused by Gram-negative organisms[41],
[42]
. The first study was a prospective, randomised, double-blind, multicentre trial (ClinicalTrials.gov identifier: NCT00690378) comparing ceftazidime 500mg-avibactam 125mg administered as a 30-minute infusion every 8 hours with imipenem-cilastatin 500mg administered as a 30-minute infusion every 6 hours for the treatment of complicated urinary tract infections (cUTIs) in hospitalised patients[41]
. Adult patients were included if they were diagnosed with acute pyelonephritis or another cUTI owing to Gram-negative organisms. Patients with a cUTI caused by an organism with known resistance to either of the study drugs were excluded. Treatment duration was 7 to 14 days (subjects were permitted to switch to oral ciprofloxacin or alternative oral therapy after a minimum of four days of intravenous therapy). The primary endpoint was microbiological response five to nine days after the last dose of study medication (test-of-cure; TOC) in the microbiologically evaluable (ME) population. Secondary endpoints included microbiological response at the end of intravenous therapy and four to six weeks post therapy (late follow-up; LFU) in the ME population, along with clinical response at the end of intravenous therapy, TOC, and LFU in the clinically evaluable (CE) population[41]
.
Overall, 137 patients were randomised to receive one of either study drug. Baseline characteristics were similar between both groups: average age ~47 years old, a primarily female population (~75%), and over 60% with a primary diagnosis of acute pyelonephritis. In the ME population, the most commonly isolated uropathogen was E. coli (>90%). Only three patients in the ceftazidime-avibactam arm and four in the imipenem-cilastatin arm had positive blood cultures; all identified as E. coli. The ME population consisted of 27 patients in the ceftazidime-avibactam group and 35 in the imipenem-cilastatin group. The CE population included 28 and 36 patients in each group respectively, and 46 and 49 patients respectively in the modified intention-to-treat (MITT) population[41]
.
The primary endpoint, favourable microbiological response at TOC in the ME population, was obtained in 19/27 (70.4%) patients in the ceftazidime-avibactam group and 25/35 (71.4%) patients in the imipenem-cilastatin group (observed difference –1.1%; 95% CI: –27.2% – 25%). Favourable microbiological response at the end of intravenous therapy was 25/26 (96.2%) and 34/34 (100%) patients respectively, and 15/26 (57.7%) and 18/30 (60%) patients respectively at LFU. A favourable clinical response in the CE population was obtained in all patients in both groups at the end of intravenous therapy. At TOC, a favourable clinical response was seen in 24/28 (85.7%) patients in the ceftazidime-avibactam group and 29/36 (80.6%) patients in the imipenem-cilastatin group; and 20/27 (74.1%) and 24/36 (66.7%) patients respectively at LFU[41]
.
Lucasti et al. conducted a phase II, prospective, randomised, double-blind, active-controlled trial (ClinicalTrials.gov identifier: NCT00752219) evaluating ceftazidime 2,000mg-avibactam 500mg administered as a 30-minute infusion every eight hours plus metronidazole 500mg infused over one hour every eight hours, versus meropenem 1,000mg every eight hours plus placebo (to maintain blinding) for the treatment of complicated intra-abdominal infections (cIAIs) in hospitalised adults[42]
. Patients with evidence of a cIAI requiring surgical intervention and antibiotics were included. Patients were excluded if the infection was caused by an organism with known resistance to the study drug, if they had an acute physiological assessment and chronic health evaluation (APACHE) II score of >25, and if they were unlikely to respond to up to 14 days of treatment. Treatment duration was 5 to 14 days; patients were permitted concomitant vancomycin, linezolid or daptomycin for suspected or documented Gram-positive infection. The primary outcome was clinical response in the ME group two weeks after the last dose of study medication (TOC). Secondary outcomes included clinical response in the CE group at the end of therapy, TOC and four to six weeks post therapy (LFU)[42]
.
Overall, 204 patients were randomised. Baseline characteristics were similar between both groups with a predominantly male population. The most common site of infection was the appendix and stomach/duodenum, with rates of surgical procedures consistent between both groups. The CE population consisted of 87 patients in the ceftazidime-avibactam plus metronidazole group and 90 in the meropenem group. The ME population included 68 and 76 patients in each group respectively[42]
.
Favourable clinical response in the ME population, the primary outcome, was observed in 62/68 (91.2%) patients in the ceftazidime-avibactam plus metronidazole group and 71/76 (93.4%) patients in the meropenem group at TOC (observed difference –2.2%; 95% CI: –20.4% – 12.2%). Within this population, 43 patients (26 ceftazidime-avibactam plus metronidazole and 17 meropenem) had pathogens that were non-susceptible to ceftazidime alone (MIC >8mg/L). All but two of these patients had a favourable microbiological response. In the CE population, a favourable clinical response at the end of therapy was seen in 84/87 (96.6%) and 87/89 (97.8%) patients respectively. These rates were only slightly lower in both groups between TOC and LFU[42]
.
More recently, three Phase III studies have been published: RECLAIM 1 and 2 (ClinicalTrials.gov Identifier: NCT01499290 and NCT01500239), RECAPTURE 1 and 2 (ClinicalTrials.gov Identifier: NCT01595438 and NCT01599806), and REPRISE (ClinicalTrials.gov Identifier: NCT01644643). These studies assessed the efficacy and safety of ceftazidime-avibactam in the treatment of cIAIs, cUTIs, and the treatment of infections due to ceftazidime-resistant Gram-negative organisms respectively[43]
[44]
,[45]
. RECLAIM was a prospective, randomised, double-blind, comparative study assessing the efficacy of ceftazidime 2,000mg-avibactam 500mg administered as a 2-hour infusion every 8 hours plus metronidazole 500mg infused over 1 hour every 8 hours, versus meropenem 1,000mg administered as a 30-minute infusion every 8 hours for the treatment of cIAIs requiring surgical intervention or percutaneous drainage[43]
. Matched placebos were administered accordingly. Patients with traumatic bowel perforation, necrotising pancreatitis, ischaemic bowel without perforation, and simple cholecystitis or appendicitis without rupture were excluded. Treatment duration was 5 to 14 days; patients were permitted concomitant vancomycin, linezolid or daptomycin for suspected or documented Gram-positive infection. Treatment could be discontinued if patients received at least five days of study drug and showed clinical signs of improvement. The primary endpoint, clinical cure at 28 to 35 days post randomisation (TOC), was assessed by non-inferiority, in the microbiologically modified intention-to-treat (mMITT), MITT and CE populations. Secondary endpoints included clinical response at the end of treatment (EOT) and 42 to 49 days post randomisation (LFU) and microbiological response at EOT, TOC, and LFU. Additionally, efficacy of ceftazidime-avibactam plus metronidazole, and meropenem alone, were assessed in patients with ceftazidime-resistant organisms[43]
.
A total of 1,066 patients were randomised to one of either study group. Baseline characteristics were similar between groups with a mean duration of therapy of 8 days. In the primary endpoint, clinical cure, ceftazidime-avibactam plus metronidazole was found to be non-inferior to meropenem in the mMITT, MITT and CE populations [82% vs 85%, –3.5; CI: –8.6 to 1.6], [83% vs 85%, –2.4; CI: –7.0 to 2.1], and [92% vs 93%, –0.8; CI: –4.6 to 2.9] respectively. Clinical and microbiological responses were not evaluated for non-inferiority; however, the results were similar to the primary endpoint. Interestingly, patients with moderate renal impairment who received ceftazidime-avibactam plus metronidazole had lower clinical cure rates compared to those who received meropenem [45% vs 74%, –29.1; CI: –50.5 to –5.4]. The authors of the study contributed this difference to the possibility of insufficient dosing in a small subset of patients with rapidly improving renal function. Clinical cure among both groups with infections due to ceftazidime-resistant and -susceptible organisms were similar[43]
.
RECAPTURE was a randomised, double-blind, double-dummy study assessing the efficacy of ceftazidime 2,000mg-avibactam 500mg administered as a two-hour infusion every eight hours versus doripenem 500mg administered as a one-hour infusion every eight hours for the treatment of cUTIs or acute pyelonephritis[44]
. Although removed from the market in Europe in 2014 due to efficacy and safety reasons in the treatment of nosocomial pneumonia, doripenem is available in the United States for treatment of cUTI and acute pyelonephritis. Patients with urinary obstruction, renal abscess, prostatitis, or Crcl 30mL/min or on haemodialysis were excluded. Treatment duration was 10 days (14 days in patients with concomitant bacteraemia). Patients were allowed to switch to oral ciprofloxacin or sulfamethoxazole-trimethroprim after ≥5 days of intravenous therapy. FDA-defined co-primary endpoints included: resolution of symptoms at day five and combined resolution of symptoms/microbiological response at TOC in the mMITT. The EMA-defined primary endpoint was microbiological response alone at TOC in the mMITT population. Secondary endpoints included microbiological response at EOT and LFU and clinical cure at EOT, TOC and LFU[44]
.
In the primary efficacy analysis a total of 810 patients were randomised to one of either study group. Baseline characteristics were similar between the groups. The majority of patients (70%) had pyelonephritis and approximately 8% had concomitant bacteraemia. E. coli was the most common pathogen isolated. In the mMITT population, ceftazidime-avibactam demonstrated non-inferiority for both FDA-defined primary endpoints [70% vs 66%, 4.0; CI: –2.4 to 10.4], [71% vs 65%, 6.7; CI: 0.3 to 13.1], and superiority for the EMA-defined primary endpoint of microbiological response at TOC [77% vs 71%, 6.4; CI: 0.3 to 12.6]. Microbiological response at LFU was higher with ceftazidime-avibactam, consistent with the findings in the EMA-defined primary endpoint at TOC. Clinical cure at EOT, TOC and LFU were similar between the groups. For ceftazidime non-susceptible pathogens, microbiological eradication was lower when compared to ceftazidime-susceptible pathogens, but similar between treatment groups [64% vs 60%, 4.0; CI: –11.1 to 18.8 and 82% vs 73%, 8.6; CI: 2.0 to 15.1]. Unlike RECLAIM, patients with impaired renal function receiving ceftazidime-avibactam did not demonstrate lower cure rates. However, renal dosing adjustments in this study differed from those used in RECLAIM owing to possible underdosing[44]
.
REPRISE was the first randomised trial that assessed the efficacy of ceftazidime-avibactam, compared to best available therapy, in patients with cUTIs or cIAIs due to ceftazidime-resistant Gram-negative organisms (Enterobacteriaceae or P. aeruginosa with intermediate or resistant ceftazidime susceptibility per CLSI criteria or resistant per EUCAST criteria)[45]
. A total of 333 patients were randomised to one of either treatment group. The majority of patients had cUTIs. Baseline characteristics (including causative pathogens and infection type) were similar between treatment groups. Ten patients with cUTIs had concomitant bacteraemia; no patients with cIAIs had bacteraemia.
The most commonly isolated organisms were Enterobacteriaceae, specifically E. coli and K. pneumoniae. Of the Enterobacteriaceae urinary isolates, 97% were ceftazidime-resistant (MIC ≥8mg/L) and 2% were presumed resistant to ceftazidime-avibactam (MIC ≥8mg/L). Of the P. aeruginosa urinary isolates, all but one was ceftazidime-resistant (MIC >16mg/L) and 9/14 (64%) were presumed resistant to ceftazidime-avibactam (MIC ≥8mg/L). All urinary isolates in the best available therapy group were susceptible to applicable therapy.
In patients with cIAIs, 95% of Enterobacteriaceae were resistant to ceftazidime and 100% were considered susceptible to ceftazidime-avibactam. In the ceftazidime-avibactam treatment group, only one P. aeruginosa isolate was presumed resistant to ceftazidime-avibactam. In the mMITT population, overall clinical cure at TOC in patients with cUTIs and cIAIs was similar between both treatment groups [91%; CI: 85.6 to 94.7 vs 91%; CI: 85.9 to 95.0]. Similar results were seen in the separate cUTI population [92%; CI: 86.3 to 95.4 vs 94%; CI: 89.3 to 97.2].
In the cIAI population, clinical cure was 8/10 (80%) in the ceftazidime-avibactam group vs 6/11 (55%) in the best available therapy group. Carbapenems (imipenem and meropenem) were the most often used in the best available therapy group. Favourable microbiological response in the cUTI population, at TOC, was higher in the ceftazidime-avibactam treatment group [82%; CI: 75.1 to 87.6 vs 64%; CI: 56 to 71.9]. In this treatment group, 7/9 patients with presumed ceftazidime-avibactam resistant P. aeruginosa had a favourable microbiological response, possibly due to the high concentrations of the drug in the urine. For patients with cIAIs, favourable microbiological response was assumed from the clinical response. However, due to the small number of patients with cIAIs, interpretation of these results is limited[45]
.
A phase III trial evaluating ceftazidime-avibactam for treatment of nosocomial pneumonia including ventilator-associated pneumonia (NCT01808092) has been completed and is awaiting publication[46]
.
Safety and tolerability
In all Phase I, II, and III studies, ceftazidime-avibactam was well tolerated[33],
[34],
[41]
[42]
,[43]
,[44]
,[45]
. In the cIAI studies, adverse events (AEs) were similar between both treatment groups; 45.9% of patients in the ceftazidime-avibactam group and 42.9% in the meropenem group, with the majority being mild to moderate in severity[42],
[43]
. Serious adverse events (SAEs) occurred in 7.9% and 7.6% of patients respectively[43]
. Types of AEs were similar between both groups; however, there were more cases of nausea, vomiting and abdominal pain in the ceftazidime-avibactam plus metronidazole group[42],
[43]
. In the cUTI studies, AEs were similar between the ceftazidime-avibactam group and doripenem group (36.2% vs 31% of patients respectively), with the most commonly reported AEs being constipation, diarrhoea, abdominal pain and headache[41],
[44]
. In the Phase II cUTI study, SAEs occurred in 4.1% and 2.4% of patients respectively, and 3/6 SAEs in the ceftazidime-avibactam group that were considered to be drug-related were renal failure, diarrhoea and accidental overdose (no AE associated with this event)[41]
.
Results from the phase III clinical trial suggest lower cure rates in patients with a baseline Crcl ≥30 – ≤50mL/min in patients treated with ceftazidime-avibactam plus metronidazole (14/31; 45%) versus those who received meropenem (26/35; 74%)[42],
[43]
. However, patients within this renal subgroup received a 33% lower daily dose than the package insert recommended renal adjustment[43]
. Further trials are warranted to assess the use in patients with renal dysfunction. Close monitoring and careful renal adjustments are recommended for this particular patient population.
Availability, dosage and administration
Ceftazidime-avibactam (Avycaz) 2.5g for injection is supplied as a single-dose vial containing ceftazidime 2g and avibactam 0.5g as a sterile powder for reconstitution[2]
. It is administered intravenously every eight hours infused over two hours in patients with a Crcl > 50mL/min. Table 4 highlights the dosing adjustments for patients with renal impairment.
| Estimated creatinine clearance (mL/min) | Dosing of ceftazidime-avibactamb |
|---|---|
| a Administer ceftazidime-avibactam after haemodialysis on haemodialysis days b Infuse all doses for two hours | |
| >50mL/min | No adjustments |
| 31–50 | 1.25g Q8H |
| 16–30 | 0.94g Q12H |
| 6–15a | 0.94g Q24H |
| ≤ 5a | 0.94g Q48H |
Conclusion
The addition of avibactam to ceftazidime broadens its spectrum of activity to include Gram-negative bacilli producing ESBLs, KPC and/or AmpC β-lactamases. Unfortunately, this combination does not enhance ceftazidime’s activity against class B β-lactamase-producing isolates, Acinetobacter spp., certain Burkholderia spp., MDR P. aeruginosa or most anaerobes. Based on Monte Carlo simulations, a 2g dose infused over two hours every eight hours has been proposed to maximise the pharmacodynamics of this agent. Current phase II studies have shown ceftazidime-avibactam to be as efficacious as carbapenems (+/- metronidazole) in the treatment of cIAIs as well as cUTIs (including acute pyelonephritis). Safety and efficacy trials have also shown this agent to be well tolerated with side effects similar to ceftazidime alone, with the most common being nausea, vomiting, constipation and anxiety. This agent offers clinicians hope in treating patients with limited to no therapeutic options. Results from phase III studies will offer insight on ceftazidime-avibactam’s efficacy in the treatment of seriously ill patients with hospital- and ventilator-acquired pneumonia and those with infections due to ceftazidime-resistant Gram-negative organisms. The development of other novel β-lactam/β-lactamase combinations, including ceftolozane-tazobactam, ceftaroline-avibactam, aztreonam-avibactam, imipenem-relebactam, biapenem-RPX7009 and meropenem-RPX7009, will help determine the exact role and place in therapy of ceftazidime-avibactam in the treatment of such infections.
Author disclosures and conflicts of interest:
Sharanie V Sims is on the speaker’s bureau for Allergan. Elizabeth A Neuner and Robert A Bonomo have no relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. No writing assistance was used in the production of this manuscript.
Reading this article counts towards your CPD
You can use the following forms to record your learning and action points from this article from Pharmaceutical Journal Publications.
Your CPD module results are stored against your account here at The Pharmaceutical Journal. You must be registered and logged into the site to do this. To review your module results, go to the ‘My Account’ tab and then ‘My CPD’.
Any training, learning or development activities that you undertake for CPD can also be recorded as evidence as part of your RPS Faculty practice-based portfolio when preparing for Faculty membership. To start your RPS Faculty journey today, access the portfolio and tools at www.rpharms.com/Faculty
If your learning was planned in advance, please click:
If your learning was spontaneous, please click:
References
[1] Department of Health. UK Five Year Antimicrobial Resistance Strategy 2013 to 2018, England: 2013. Available at: https://www.gov.uk/government/publications/uk-5-year-antimicrobial-resistance-strategy-2013-to-2018 (accessed May 2017).
[2] Avycaz [package insert]. Forest Pharmaceuticals, Inc., Cincinnati, OH; September 2015.
[3] Infectious Diseases Society of America. The 10 x ’20 initiative: pursing a global commitment to develop 10 new antibacterial drugs by 2020. Clin Infect Dis 2010;50:1081. doi: 10.1086/652237
[4] Boucher HW, Talbot GH, Benjamin Jr DK et al. 10 x ’20 progress – development of new drugs active against gram-negative bacilli: an update from the infectious diseases society of America. Clin Infect Dis 2013;56(12):1685–1694. doi: 10.1093/cid/cit152
[5] Centers for Disease Control and Prevention (CDC). Antibiotic resistance threats in the United States, 2013. Atlanta: CDC; 2013. Available at: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf (accessed May 2017).
[6] Zhanel G, Lawson C, Adam H et al. Ceftazidime-Avibactam: a novel cephalosporin/ß-lactamase inhibitor combination. Drugs 2013;73:159–177. doi: 10.1007/s40265-013-0013-7
[7] Neu HC & Labthavikul P. Antibacterial activity and beta-lactamase stability of ceftazidime, an aminothiazolyl cephalosporin potentially active against pseudomonas aeruginosa. Antimicrob Agents Chemother 1982;21:11–18. doi: 10.1128/AAC.21.1.11
[8] Garzone P, Lyon J & Yu VL. Third-generation and investigational cephalosporins: I. structure-activity relationships and pharmacokinetic review. Drug Intell Clin Pharm 1983;17:507. doi: 10.1177/106002808301700703
[9] Stachyra T, Pechereau MC, Bruneau JM et al. Mechanistic studies of the inactivation of TEM-1 and P99 by NXL104, a novel non-beta-lactam beta-lactamase inhibitor. Antimicrob Agents Chemother 2010;54:5132–5138. doi: 10.1128/AAC.00568-10
[10] Ehmann DE, Jahic H, Ross PL et al. Avibactam is a covalent, reversible, non-beta-lactam beta-lactamase inhibitor. Proc Natl Acad Sci USA 2012;109:11663. doi: 10.1073/pnas.1205073109
[11] Clinical and Laboratory Standards Institute. M100-S26: Performance standards for antimicrobial susceptibility testing. Twenty-sixth edition. Wayne, PA: CLSI; 2016.
[12] European Committee on Antimicrobial Susceptibility Testing (EUCAST). [Intranet]. Available at: http://www.eucast.org (accessed May 2017).
[13] Levasseur P, Girard AM, Miossec C et al. In vitro antibacterial activity of the ceftazidime-avibactam combination against enterobacteriaceae, including strains with well characterized beta-lactamases. Antimicrob Agents Chemother 2015; 59(4):1931–1934. doi: 10.1128/AAC.04218-14
[14] Castanheira M, Farrell SE, Krause KM et al. Contemporary diversity of beta-lactamases among Enterobacteriaceae in the nine U.S. census regions and ceftazidime-avibactam activity tested against isolates producing the most prevalent beta-lactamase groups. Antimicrob Agents Chemother 2014;58:833–888. doi: 10.1128/AAC.01896-13
[15] Endimiani A, Choudhary Y & Bonomo RA. In vitro activity of NXL104 in combination with beta-lactams against klebsiella pneumoniae isolates producing KPC carbapenemases. Antimicrob Agents Chemother 2009;53:3599–3601. doi: 10.1128/AAC.00641-09
[16] Aktas Z, Kayacan C & Oncul O. In vitro activity of avibactam (NXL104) in combination with beta-lactams against gram-negative bacteria, including OXA-48 beta-lactamase-producing klebsiella pneumoniae. Int J Antimicrob Agents 2012;39:86–89. doi: 10.1016/j.ijantimicag.2011.09.012
[17] Ehmann DE, Jahic H, Ross PL et al. Kinetics of avibactam inhibition against Class A, C, and D beta-lactamases. J Biol Chem 2013;288:27960–27971. doi: 10.1074/jbc.M113.485979
[18] de Jonge BLM, Karlowsky JA, Kazmierczak KM et al. In vitro susceptibility to ceftazidime-avibactam of carbapenems-nonsusceptible enterobacteriaceae isolates collected during the INFORM global surveillance study (2012-2014). Antimicrob Agents Chemother 2016;60(5):3163–3169. doi: 10.1128/AAC.03042-15
[19] Mushtaq S, Warner M & Livermore DM. In vitro activity of ceftazidime+NXL104 against Pseudomonas aeruginosa and other non-fermenters. J Antimicrob Chemother 2010;65:2376–2381. doi: 10.1093/jac/dkq306
[20] Flamm RK, Sader HS, Farrell DJ et al. Ceftazidime-avibactam and comparator agents tested against urinary tract isolates from a global surveillance program (2011). Diagn Microbiol Infect Dis 2014;80:233–238. doi: 10.1016/j.diagmicrobio.2014.07.005
[21] Citron DM, Tyrrell KL, Merriam V et al. In vitro activity of ceftazidime-NXL104 against 396 strains of beta-lactamase-producing anaerobes. Antimicrob Agents Chemother 2011;55:3616–3620. doi: 10.1128/AAC.01682-10
[22] Karlowsky JA, Biedenbach DJ, Kazmierczak KM et al. Activity of ceftazidime-avibactam against extended-spectrum- and ampC B-lactamase-producing enterobacteriaceae collected in the INFORM global surveillance study from 2012 to 2014. Antimicrob Agents Chemother 2016;60(5):2849–2857. doi: 10.1128/AAC.02286-15
[23] Nichols WW, de Jonge BLM, Kazmierczak KM et al. In vitro susceptibility of global surveillance isolates of Pseudomonas aeruginosa to ceftazidime-avibactam (INFORM 2012 to 2014). Antimicrob Agents Chemother 2016;60(8):4743–4749. doi: 10.1128/AAC.00220-16
[24] Sader HS, Castanheira M, Farrell DJ et al. Ceftazidime-avibactam activity when tested against ceftazidime-nonsusceptible citrobacter spp., enterobacter spp., serratia marcescens, and Pseudomonas aeruginosa from United States medical centers (2011-2014). Diagn Microbiol Infect Dis 2015(83):389–394. doi: 10.1016/j.diagmicrobio.2015.06.008
[25] Flamm RK, Farrell DJ, Sader HS et al. Ceftazidime/avibactam activity tested against gram-negative bacteria isolated from bloodstream, pneumonia, intra-abdominal and urinary tract infections in US medical centres (2012). J Antimicrob Chemother 2014;69:1589–1598. doi: 10.1093/jac/dku025
[26] Livermore DM, Mushtaq S, Warner M, et al. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2011;55(1):390–394. doi: 10.1128/AAC.00756-10
[27] Winkler ML, Papp-Wallace KM, Hujer AM et al. Unexpected challenges in treating multidrug-resistant gram-negative bacteria: resistance to ceftazidime-avibactam in archived isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother 2015;59:1020–1029. doi: 10.1128/AAC.04238-14
[28] Humphries RM, Yang S, Hemarajata P et al. First report of ceftazidime-avibactam resistance in a KPC-3-expressing klebsiella pneumoniae isolate. Antimicrob Agents Chemother 2015;59:6605–6607. doi:10.1128/AAC.01165-15
[29] Lahiri SD, Giacobbe RA, Johnstone MR et al. Activity of avibactam against enterobacter cloacae producing an extended-spectrum class C beta-lactamase enzyme. J Antimicrob Chemother 2014;69:2942–2946. doi: 10.1093/jac/dku237
[30] Lahiri SD, Walkup GK, Whiteaker JD et al. Selection and molecular characterization of ceftazidime/avibactam-resistant mutants in Pseudomonas aeruginosa strains containing derepressed AmpC. J Antimicrob Chemother 2015;70:1650–1658. doi: 10.1093/jac/dkv004
[31] Craig W & Andes D. Cephalosporins. In: Bennett J, Dolin R & Blaser M, editors. Mandell, Douglas, and Bennett’s Principles and practice of infectious diseases. 8th ed. Philadelphia: Churchill Livingstone Elsevier; 2015: p. 278–292.
[32] Richards D & Brogden R. Ceftazidime, a review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1985;29:105–161. doi: 10.2165/00003495-198529020-00002
[33] Merdjan H, Rangaraju M & Tarral A. Safety and pharmacokinetics of single and multiple ascending doses of avibactam alone and in combination with ceftazidime in healthy male volunteers: results of two randomized, placebo-controlled studies. Clin Drug Investig 2015;35:307–317. doi: 10.1007/s40261-015-0283-9
[34] Tominaga N, Edeki T, Li James et al. Phase I study assess the safety, tolerability, and pharmacokinetics of avibactam and ceftazidime-avibactam in healthy Japanese volunteers. J Infect Chemother 2015;21:551–558. doi: 10.1016/j.jiac.2015.04.006
[35] Boselli E, Breilh D, Rimmele T et al. Plasma and lung concentrations of ceftazidime administered in continuous infusion to critically ill patients with severe nosocomial pneumonia. Intensive Care Med 2004;30(5):989–991. doi: 10.1007/s00134-004-2171-2
[36] Zasowski EJ, Rybak JM & Rybak MJ. The β-lactams strike back: ceftazidime-avibactam. Pharmacotherapy 2015;35(8):755–770. doi: 10.1002/phar.1622
[37] Merdjan H, Tarral A, Girard A et al. Safety, single dose pharmacokinetics, and pharmacodynamics of β-lactamse inhibitor NXL104 in healthy young male adults [abstract no. A-809 plus poster]. In: 47th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sep 17-20; Chicago (IL).
[38] Coleman K, Levasseur P, Girard AM et al. Activities of ceftazidime and avibactam against β-lactamase-producing Enterobacteriaceae in a hollow-fiber pharmacodynamics model. Antimicrob Agents Chemother 2014;58(6):3366–3372. doi: 10.1128/AAC.00080-14
[39] Boselli E, Breilh D, Rimmele T et al. Plasma and lung concentrations of ceftazidime administered in continuous infusion to critically ill patients with severe nosocomial pneumonia. Intensive Care Med 2004;30(5);989–991. doi: 10.1007/s00134-004-2171-2
[40] Berkhout J, Melchers M, van Mil A et al. Pharmacodynamics of ceftazidime and avibactam in neutropenic mice with thigh or lung infection. Antimicrob Agents Chemother 2015;60(1):368–375. doi: 10.1128/AAC.01269-15
[41] Vazquez J, Gonzalez Patzan L, Stricklin D et al. Efficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: results of a prospective, investigator-blinded, randomized study. Curr Med Res Opin 2012;28(12):1921–1931. doi: 10.1185/03007995.2012.748653
[42] Lucasti C, Popescu I, Ramesh M et al. Comparative study of the efficacy and safety of ceftazidime/avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infections in hospitalized adults: results of a randomized, double-blind, phase II trial. J Antimicrob Chemother 2013;68:183–192. doi: 10.1093/jac/dks523
[43] Mazuski JE, Gasink LB, Armstrong J et al. Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis 2016;62(11):1380–1389. PMCID: PMC4996135
[44] Wagenlehner FM, Sobel JD, Newell P et al. Ceftazidime-avibactam versus doripenem for the treatment of complicated urinary tract infections, including acute pyelonephritis: RECAPTURE, a phase 3 randomized trial program. Clin Infect Dis 2016;63(6):754–762. PMCID: PMC4996135
[45] Carmeli Y, Armstrong J, Laud PJ et al. Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed, phase 3 study. Lancet Infect Dis 2016;16:661–673. doi: 10.1016/S1473-3099(16)30004-4
[46] Ceftazidime avibactam clinical trials. https://clinicaltrials.gov/ct2/results?term =ceftazidime +avibactam&Search=Search (accessed May 2017).