This content was published in 2003. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
Identify gaps in your knowledge
- List three risk factors for atrial fibrillation.
- What are the options for managing atrial fibrillation?
- What prevents the long-term use of amiodarone?
Before reading on, think about how this article may help you to do your job better.
The Royal Pharmaceutical Society’s areas of competence for pharmacists are listed in “Plan and record,” (available at: www.rpsgb.org.uk/education). This article relates to “common disease states and their drug therapies” (see appendix 4 of “Plan and record”).
The first part of this article (PJ, 20 September) outlined the mechanisms controlling normal heart rate and rhythm, and the management of bradycardias and some atrial tachycardias. This second part focuses onthe management of atrial fibrillation and ventricular tachycardia.
ATRIAL FIBRILLATION
Atrial fibrillation (AF) is the most commonly occurring supraventricular tachycardia — present in one in 20 people over the age of 65 years in the United Kingdom. The condition is not acutely life threatening, but neither should it be considered benign — AF is a major cause of morbidity and almost doubles mortality. The main danger is a five-fold increase in stroke and many studies have commented that better treatment of AF could substantially reduce the burden of illness in society, particularly through stroke prevention.
The incidence of AF increases with age and it is commonly caused by hypertension, ischaemic heart disease or structural heart diseases (eg, cardiomyopathy and constrictive pericarditis). Non-cardiac risk factors include diabetes, thyrotoxicosis, high alcohol intake and chronic obstructive airways disease. Despite AF being responsible for most arrhythmia-related hospital admissions, only about one-third of cases ever present to hospital, suggesting that many cases can be successfully managed in primary care.
Description
AF is characterised by an irregular, rapid atrial rate (usually between 300 and 600bpm), which occurs secondary to chaotic conduction of electrical impulses within the atria. Uncoordinated, small, multiple re-entry circuits (localised circling of electrical impulses) lead to asynchronous electrical activity, loss of atrial systolic function and an irregular ventricular response. The ventricular rate in AF is limited by the atrioventricular (AV) node’s inability to conduct these disorganised atrial impulses and does not usually exceed 180bpm.
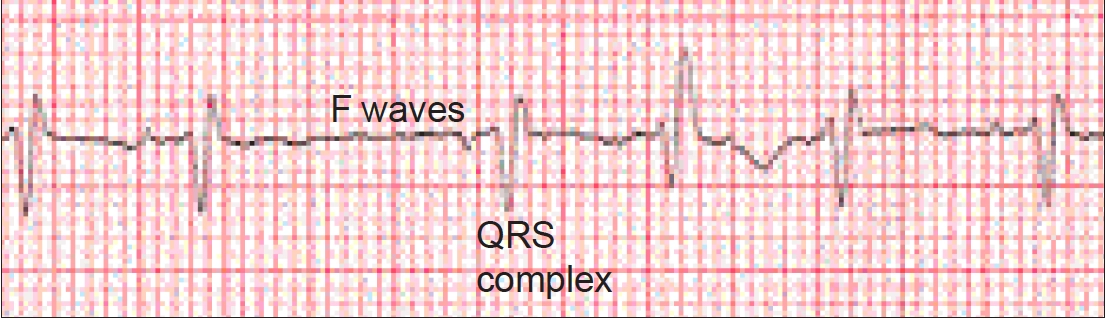
Diagnosis of AF should be made using an electrocardiogram (ECG), which shows an absence of consistent P waves (indicating the lack of organised atrial activity) before each QRS complex. P waves are replaced by “f” or “fibrillation” waves; rapid oscillations varying in size, shape and timing. The ECG will also demonstrate the irregular ventricular rate (see Figure 1).
AF is generally described as either acute (present for less than 48 hours) or chronic (persisting for more than 48 hours). Chronic AF can be further classified as paroxysmal, persistent or permanent (see Panel 1).
Panel 1: Classification of chronic atrial fibrillation
Paroxysmal atrial fibrillation Paroxysmal atrial fibrillation (PAF), also called “intermittent” or “self-terminating”, describes a condition where the individual experiences repeated episodes of fibrillation interspersed with periods of sinus rhythm. Episodes can vary in both duration and frequency lasting for, or occurring at, intervals of a few seconds to several days
Persistent atrial fibrillation Persistent AF occurs where fibrillation has been shown to last for more than 48 hours, but may be successfully converted to sinus rhythm using electrical or pharmacological interventions
Permanent atrial fibrillation Permanent AF describes episodes that last for more than 48 hours and where attempts to restore sinus rhythm have failed or where such procedures are unsuitable for the patient
Symptoms
Although AF can be asymptomatic, most patients experience chest pain, shortness of breath, dizziness or fatigue. Other symptoms include general ill health, low exercise tolerance, irritability, poor concentration and palpitations (at rest or precipitated by exercise or emotion, or both), all of which may decrease quality of life. In some cases AF can precipitate episodes of angina or may present as acute heart failure. In addition to the symptoms described, AF has the potential to cause serious morbidity by:
- Worsening existing heart failure through reducing cardiac output (atrial contractions are disordered and ventricular fillingis impaired) and changing fluid balance. This can lower blood pressure or lead to pulmonary oedema or symptoms of angina
- Increasing the risk of stroke. Stasis of blood within the atria predisposes to cerebral and systemic thromboembolism. Sluggish atrial blood flow also allows partial activation of the clotting cascade, further increasing the risk of thrombosis
Management strategies
The traditional aim of managing AF is to restore sinus rhythm (electrically or pharmacologically). Restoration and maintenance of sinus rhythm relieves symptoms, improves cardiac output and prevents the development of cardiomyopathy. Another objective is to control the ventricular rate, to minimise the haemodynamic consequences. Investigating and addressing any cause or precipitating factor for AF is also essential. For example, restoring normal thyroid function in the context of thyrotoxic acute AF often results in spontaneous restoration of sinus rhythm in many patients.
The strategies chosen to treat a patient will depend primarily on symptom severity and the characteristics of the AF. Table 1 gives a summary of anti-arrhythmic drugs used. Prevention of thromboembolism is also an important management strategy (see below).
| Aims | Class of drugs used and examples |
| Cardioversion to sinus rhythm | Ia (eg, quinidine, procainamide, isopyramide), Ic (eg, flecainide, propafenone), III (eg, amiodarone, sotalol) |
| Maintenance of sinus rhythm post cardioversion | Ia, Ic, II (eg, beta-blockers), III |
| Management of PAF | Ia, Ic, II, III |
| Rate control | II, III, IV (eg, verapamil, diltiazem), digoxin |
Management of acute or recent-onset atrial fibrillation
Patients who present with acute or recent-onset AF associated with significant haemodynamic compromise, such as symptomatic hypotension, acute heart failure, unstable angina, loss of consciousness or shock need urgent intervention to slow the ventricular rate and, ideally, to restore sinus rhythm. Spontaneous restoration of sinus rhythm can occur in up to 50 per cent of patients but for those in whom it does not occur drug therapy or, more commonly, electrical cardioversion may be tried. Before cardioversion, the doctor must be certain (from the patient’s clinical history) that the AF is of recent onset so that the presence of atrial thrombi can be excluded (it takes several hours for a clot to form). These patients will not need to be given anticoagulants before and after the procedure (see below) but short-term intravenous heparin is recommended. Associated complications, such as heart failure, should be managed appropriately. Patients who are haemodynamically stable and asymptomatic may be managed with a strategy of heart rate control and anticoagulation as an alternative to early electrical cardioversion, allowing time for assessment and evaluation.
Management of long-term atrial fibrillation
The incidence of chronic AF in the UK has been estimated as 1.7 per 1,000 person years.
Paroxysmal atrial fibrillation
In paroxysmal AF (PAF), the aim of therapy is to reduce the frequency of, or prevent, paroxysms and to control the ventricular rate so that haemodynamic compromise is minimal. If symptoms are mild and infrequent it is advisable not to use anti-arrhythmic therapy to avoid adverse effects. General measures, such as abstinence from alcohol and caffeine, which may precipitate paroxysms, or anti-stress counselling, should always be considered.
Where therapy is needed, the class III agent amiodarone may be particularly useful because it can reduce the frequency and duration of paroxysms. It also slows the atrial rate during paroxysms and slows AV nodal conduction (and therefore the ventricular response) during episodes of PAF. However, adverse effects, including corneal micro-deposits, pulmonary fibrosis, disturbances in thyroid function and hepatotoxicity, may limit long-term use. Sotalol, a beta-blocker with additional class III effects, is a suitable alternative but pro-arrhythmic effects can be a problem.
Class I agents may also be useful, despite the widespread belief that they are associated with increased sudden death. In the post-myocardial infarction CAST study1 a three-fold increase in mortality was seen in patients given flecainide for ventricular tachycardias. This increase is not significant in patients with structurally normal hearts, but in patients with ischaemic heart disease or cardiomyopathy (where the risk of sudden death is high), these drugs are best avoided.
Ventricular rate can be controlled with other beta-blockers (eg, atenolol, metoprolol) or rate-controlling calcium channel blockers (particularly verapamil). Digoxin is unsuitable because it can result in more frequent, rapid and persistent paroxysms. Also, direct current (DC) cardioversion is unlikely to be of use because even if sinus rhythm is achieved most patients will rapidly revert to AF.
The risk of thromboembolism is greater in patients who frequently and spontaneously revert into and out of AF and, in most cases of PAF, long-term antithrombotic therapy is warranted.
Persistent atrial fibrillation
Patients with persistent AF should be assessed for suitability for cardioversion. The choice as to which type of cardioversion (pharmacological or electrical) and, if appropriate, the type of anti-arrhythmic drug depends on the urgency of the condition, any underlying heart disease and, often, the experience or preference of the doctor. The gold standard, DC cardioversion, should be considered for all patients with new-onset AF, or where the duration of AF is less than 12 months. Early success rates are good, with 70 to 90 per cent of patients achieving sinus rhythm. Unfortunately, reversion to fibrillation is common, especially where there is underlying heart disease. The agents used to manage PAF (discussed above) may be used pre and post DC cardioversion of persistent AF to help maintain sinus rhythm. Class II and III agents can also be used to convert persistent AF to sinus rhythm, but these are generally less successful than electrical cardioversion.
Permanent atrial fibrillation
In permanent AF the aim is to control heart rate and to use antithrombotic therapy appropriately. Drug therapy is used to slow conduction across the AV node, aiming for a resting ventricular rate of 60 to 80bpm.
In permanent AF, digoxin is traditionally used to manage ventricular response. It is highly effective but limited by its narrow therapeutic range. Factors predisposing to toxicity include renal impairment, increasing age, interacting drug therapy, hypokalaemia and hypothyroidism. Digoxin cannot maintain adequate rate-control during exercise and it is therefore less suitable for more active (younger) patients. Alternatives include amiodarone, sotalol, other beta-blockers (class II) and rate-controlling calcium channel blockers (class IV). The choice of agent generally depends on the presence of co-morbidities. For example, in ischaemic heart disease or as part of a heart failure management plan, beta-blockers are an appropriate first-line option. Consideration must also be given to long-term tolerability. For example, class III agents have a higher incidence of significant adverse effects and may therefore be less suitable for long-term use.
Where electrical cardioversion and drug therapy have both failed to control AF and give an appropriate quality of life for the patient, radio frequency ablation of the AV node (see PJ, 20 September) combined with insertion of a pacemaker may be considered. In this situation, the atria continue fibrillating, but there is no conduction between the atria and the ventricles, the latter being controlled by the pacemaker unit. However, anticoagulation is still required, because the atrial rhythm is unchanged.
Antithrombotic therapy
During AF, the atria fail to contract in a co-ordinated fashion, resulting in stasis of blood within the atrial chambers. This can lead to the formation of clots, which can enter the arterial system from the left atria, resulting in stroke or other systemic thromboembolism. The use of antithrombotic therapy is therefore recommended in patients with AF.
A number of studies have investigated both primary and secondary prevention of stroke in patients with AF. Meta-analysis2 has concluded that warfarin (aiming for an international normalised ratio of 2 to 3) is the most effect antithrombotic, reducing the risk of stroke by about 60 per cent. Aspirin is less effective, reducing risk by about 20 per cent, and trial data have failed to establish an optimal dose for aspirin in stroke prevention.
Currently antithrombotic strategies are under-prescribed inpatients with AF, possibly due to fears of adverse effects, particularly older patients. Treatment should be targeted at patients likely to gain the most benefit and Panel 2 contains guidelines for treatment choice. These call for regular risk assessments.
Panel 2: Practical guidelines for antithrombotic therapy in AF*
High risk
- All patients with previous transient ischaemic attack or cerebrovascular accident (stroke)
- All patients 75 years with diabetes or hypertension
- All patients with clinical evidence of valve disease, heart failure, thyroid disease, or impaired left ventricular function
Treat with warfarin (INR 2 to 3) unless contraindicated
Moderate risk
- All patients <65 years with clinical risk factors: diabetes, hypertension, peripheral vascular disease, ischaemic heart disease
- All patients >65 years not in a high risk group
Treat with warfarin (INR 2 to 3) or aspirin (75–300mg daily) and assess on an individual case basis and consider the risk of haemorrhage, compliance issues, patient preference, etc
Low risk
- All patients <65 years with no history of embolism, hypertension, diabetes, or other clinical risk factors
Treat with aspirin (75–300mg daily)
Adequate anticoagulation is required before and after cardioversion in all patients with AF of more than 48 hours, or where the duration of AF is unknown. Cardioversion in these patients carries a 5 per cent risk of thromboembolic complications. Anticoagulation is recommended for at least three weeks before and for four weeks after the procedure. Warfarin is frequently prescribed, aiming for an INR of 2 to 3.
VENTRICULAR TACHYCARDIAS
It is common for people to experience occasional, extra ventricular beats (“ectopic beats”), but frequent, unusually shaped ventricular ectopic beats, or runs of them, are a cause for concern because they are associated with increased mortality in patients with structural heart disease. Ventricular tachycardia is defined as five or more ventricularectopic beats occurring consecutively. Ventricular rates of between 120 and 250bpm are usually seen on ECGs. Myocardial ischaemiawith a history of acute myocardial infarction is the most commonunderlying condition and mortality rates of 20 per cent over two yearshave been observed in these patients. Other causes of ventricular tachycardia include cardiomyopathies, myocarditis and valve disease.
The ECG diagnosis of ventricular tachycardia can be difficult because some features are similar to those seen in supraventricular tachycardias. If in doubt, it is safest to assume that the arrhythmia is ventricular tachycardia unless proven otherwise. The urgency of treatment depends on the severity of symptoms — some patients will rapidly develop shock or deteriorate into ventricular fibrillation (a form of cardiac arrest), while others may experience minimal or no overt symptoms. DC cardioversion should be considered where there is significant haemodynamic compromise, otherwise anti-arrhythmic drugs can be used to revert to sinus rhythm.
Amiodarone and lidocaine are most commonly prescribed for the acute management of ventricular tachycardia in the UK. Following the acute phase, a thorough investigation is needed toidentify underlying causes and additional therapy, to protect thepatient against further episodes, should be considered. However, long-term drug therapy in patients with ventricular tachycardia and heart disease is associated with increased mortality because of proarrhythmic effects, so other options are preferred. Implantable “cardioverter” defibrillators (ICDs) are devices that can monitor and treat both bradycardia and tachycardia. If a patient suffers anepisode of ventricular tachycardia, the device will automatically sense it, and initially pace the heart to control the rhythm. If this fails, or if the patient deteriorates into ventricular fibrillation the device will deliver a low voltage shock to attempt to revert to sinus rhythm. ICDs have been shown to reduce mortality by up to 30 percent, and their use in patients with sustained ventricular tachycardia causing haemodynamic compromise has been endorsed by the National Institute for Clinical Excellence.3
Despite the use of ICDs, prophylactic anti-arrhythmic therapy may still be required to control the frequency with which ventricular tachycardia occurs. Beta-blockers are commonly used in patients for heart failure and ischaemic heart disease and therefore may be the most appropriate choice for suppressing ventricular tachycardia. Otherwise, amiodarone is the agent of choice because it is mortality-neutral in patients with heart disease (with or without left ventricular dysfunction). Long-term therapy, however, is associated with significant adverse effects (discussed above). Where amiodarone is unsuccessful, class I drugs should be considered under the supervision of a specialist centre. Radiofrequency ablation may beused in some cases. In addition to treating the arrhythmia, management of the underlying disorder, for example, revascularisation and secondary prevention strategies (eg, prescribing aspirin, a statin oran angiotensin converting enzyme inhibitor) in the presence of heart disease is required.
Torsades de pointes
Torsades de pointes (“twisting of the points”) is a type of ventricular tachycardia that gives a classic (polymorphic) appearance on ECGs. Frequently self-limiting, it can be caused by drugs and every effort should be made to identify and eliminate any potential trigger. Precipitating agents include tricyclic antidepressants, erythromycin, and some antihistamines. Initial treatment consists of magnesium supplementation and temporary pacing (although urgent defibrillation may be necessary if torsades is prolonged or leads to the development of ventricular fibrillation). Anti-arrhythmic drug therapy may be needed to prevent recurrence, but some agents can exacerbate the situation, particularly class IA (procainamide) and class III (amiodarone and sotalol) agents. ICDs may be appropriate in patients with persistent conduction defects.
Action: practice points
Reading is only one way to do CPD and the Society will expect to see various approaches to CPD in a pharmacist’s portfolio.
- Make sure you understand all the terms in this article.
- Explain atrial fibrillation to a member of your team.
- Review the mechanisms of action of the drugs in this article.
Evaluate
For your work to be presented as CPD, you need to evaluate your reading and any other activities. Answer the following three questions:
What have you learnt?
How has it added value to your practice? For example, have you applied this learning or had any feedback?What will you do now and how will this be achieved?
References
- Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH et al. Mortality and morbidity in patients receiving encainide, flecainide or placebo (CAST). N Engl J Med 1991;324:781–8.
- Van Walraven C, Hart RG, Singer DE, Laupacis A, Connolly S, Petersen P, et al. Oral anticoagulants vs aspirinin non-valvular atrial fibrillation: an individual patientmeta-analysis. JAMA 2002;288:2441–8.
- National Institute for Clinical Excellence. Guidance on the use of implantable cardioverter defibrillators for arrhythmias. Available at: www.nice.org.uk/pdf/Defibrillators_A4_summary.pdf (accessed 1 October 2003).*Adapted from Lip GHY, Hart RG and Conway DSG. BMJ 2002;325:1022–5.


