This content was published in 2013. We do not recommend that you take any clinical decisions based on this information without first ensuring you have checked the latest guidance.
In short
Dabigatran, rivaroxaban and apixaban are the new oral anticoagulants. They come with a different set of risks and benefits compared with warfarin, and they represent an alternative for some patients who might not previously have been suitable for treatment with warfarin.
Patients should be counselled on the importance of adherence to therapy, how to take their medicines, certain medicines that need to be avoided and being alert for signs of bleeding.
In the past two years, three new oral anticoagulants (NOACs) have been licensed in Europe and approved by the National Institute for Health and Care Excellence for a handful of indications, including primary and secondary prevention of stroke in patients with atrial fibrillation (AF).
NOACs are at least as effective as warfarin at preventing stroke and, unlike with warfarin, patients do not need to have their international normalised ratios (INRs) monitored. NOACs also offer an advantage to patients who have problems adhering to variable dose regimens or in whom comorbidities make it difficult to achieve good INR control. This means that there are now more AF patients who can benefit from the stroke-reducing effects of anticoagulation.
However, without the need for INR monitoring for patient taking NOACs, there are fewer opportunities for healthcare professionals to provide information and reinforce the need for adherence. It is therefore important for pharmacists in all settings to make the most of any opportunities to counsel patients who are prescribed NOACs.
NOAC selection and dosing
The NOACs are divided into two therapeutic classes: the direct factor Xa inhibitors rivaroxaban and apixaban; and the direct thrombin inhibitor dabigatran.
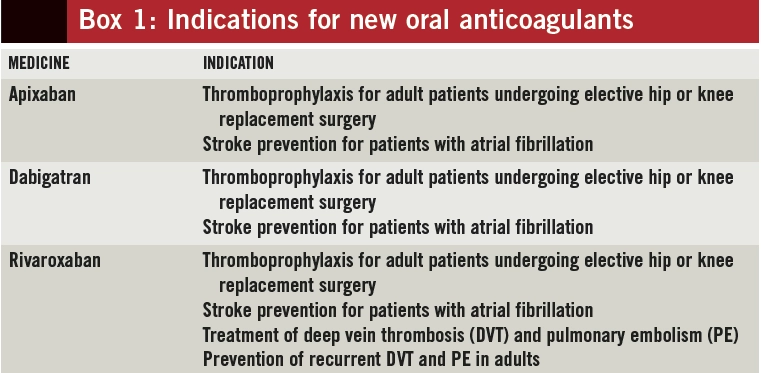
The licensed indications for NOACs, and corresponding doses, vary (see Box 1); therefore, knowing an indication for treatment is essential to ensure appropriate drug choice and dosing for each patient (use of NOACs for prevention of stroke in AF is the main focus of this article).
All NOACs are excreted to varying degrees by the kidneys. Product manufacturers provide guidance on dosing in renal impairment according to creatinine clearance as calculated using the Cockroft and Gault formula. Estimated glomerular filtration rate (eGFR), which is reported by pathology laboratories, uses population data (age, sex and ethnicity) that do not consider differences in muscle mass, and so is not suitable for guiding NOAC dosing. The Cockroft and Gault formula takes into account patient weight — allowing for more accurate assumption to be made for muscle mass. Ideal body weight should be used for obese patients.
Patient counselling
It is important that patients understand why they need to take anticoagulants and the importance of adherence. A recent meta-analysis of adherence to cardiovascular therapy demonstrated that around 9% of all cardiovascular events across Europe could be attributed to non-adherence.1
For patients with AF, pharmacists can explain that the condition is one of the leading causes of stroke and that most patients do not have warning symptoms before a stroke occurs. When communicating the results of risk assessments (eg, CHA2DS2VASc), it can help to describe percentages in terms of the number of people out of 100 who would have a stroke without anticoagulation.
Patients taking aspirin as an antiplatelet might require an explanation that full anticoagulation is considerably better than aspirin for preventing stroke.
Administration
Dabigatran capsules are relatively large and contain tartaric acid to increase the solubility of the active ingredient; capsules should be swallowed whole because removing the contents from the capsule increases bioavailability substantially. To protect the medicine from moisture, dabigatran capsules need to be stored in their original packaging and should not be added to multi-compartment compliance aids. The aluminium foil packaging is thick and capsules cannot be pushed through the foil but rather the foil needs to be peeled back to release the capsule. This requires a certain level of dexterity and may be difficult for frail or elderly patients.
Rivaroxaban and apixaban are small, film-coated tablets (red and white, respectively). Both can be crushed or added to water to form a slurry, which can be administered through an enteral feeding tube if necessary.
Missed doses
The NOACs have short half-lives (less than 24 hours) compared with warfarin (about 37 hours), so missing a NOAC dose could increase patients’ risk of stroke due to insufficient anticoagulation. The importance of not missing doses needs to be emphasised to patients.
If a dose of once-daily rivaroxaban is missed, patients can take it within 12 hours. Missed doses of twice-daily apixaban and dabigatran need to be taken within six hours. After these times, patients should omit the dose and take their next dose as scheduled.
Interactions
Drug interactions that can cause reduced or increased NOAC blood concentrations have the potential to lower treatment efficacy or increase patients’ risk of bleeding, respectively.
A complete medication history is needed before starting NOACs. Patients should also be advised to ask their pharmacist or specialist before starting any new medicines — prescribed or bought over the counter. It may not always be possible to stop or reduce doses of some interacting medicines and risk versus benefit will need to be assessed.
Rivaroxaban and apixaban should not be taken with strong inhibitors or inducers of cytochrome P450 isoenzyme CYP3A4 or P-glycoprotein (P-gp). Dabigatran should not be administered with strong P-gp inhibitors or inducers.
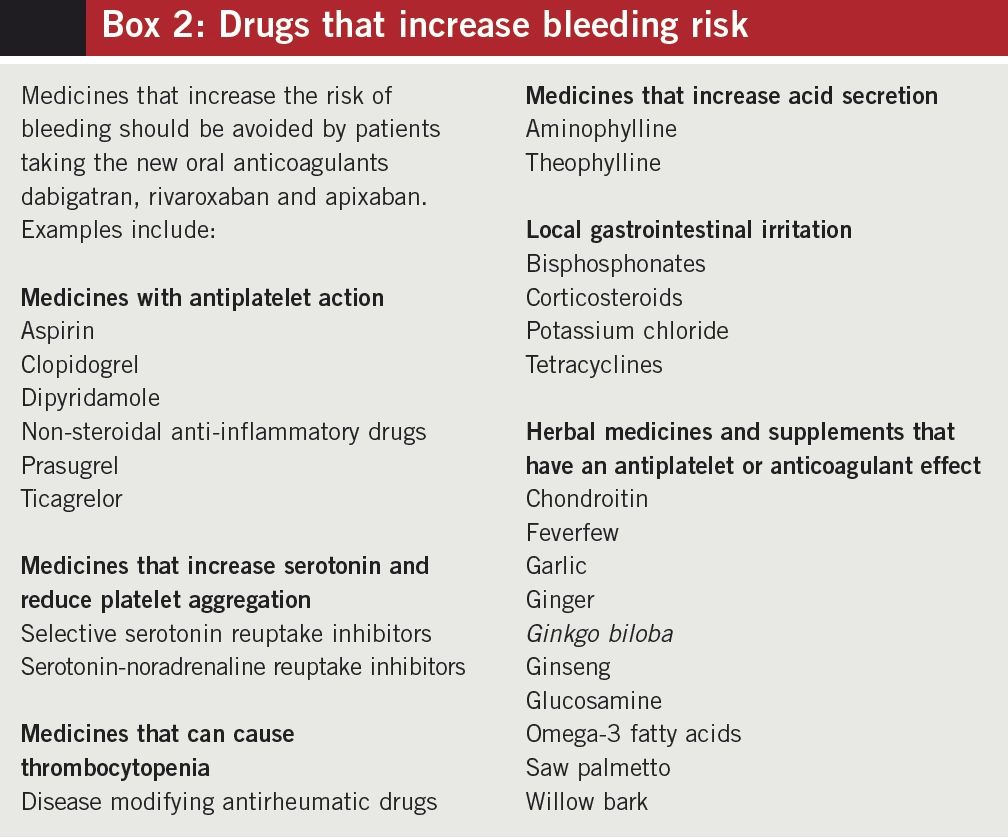
Examples of medicines and supplements that can increase the risk of bleeding in patients taking NOACs are listed in Box 2.
Food and alcohol
NOACs are not affected by vitamin K; therefore foods containing this do not need to be regulated as for patients taking warfarin. Any alcohol intake should be moderate, mainly because alcohol increases production of gastric acid, which can irritate the gastric mucosa.
Alert cards
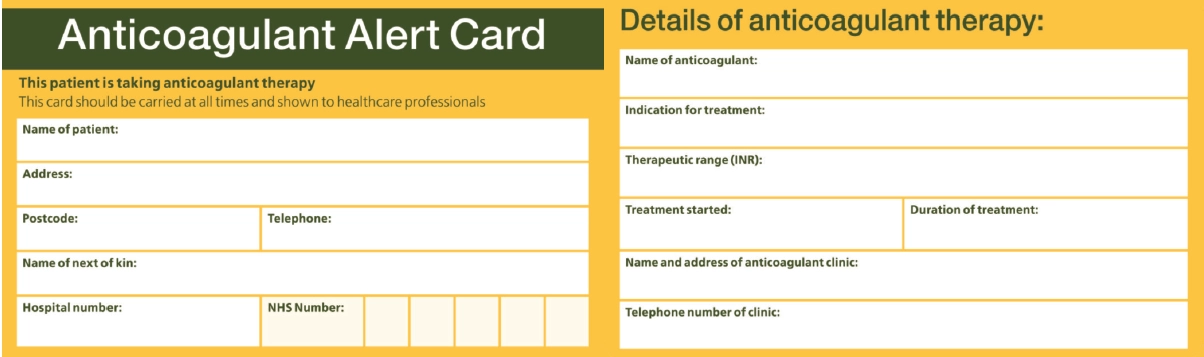
All patients who are prescribed NOACs should be given an anticoagulant alert card.
Medicine-specific cards are produced by the product manufacturers and are supplied with patient support packs. However, many healthcare professionals are unfamiliar with NOACs and do not always immediately recognise them as being anticoagulants. To assist with this, it may be more appropriate to provide all patients with the standard yellow alert card for oral anticoagulants (used routinely by patients taking warfarin; see Figure), which is widely recognised by healthcare staff (paramedics, GP receptionists, dentists, etc).
Dental procedures
Routine check-ups or having fillings by a dentist do not usually require anticoagulant cessation. Manufacturers do not offer any specific advice for dental extractions or cleaning by a hygienist; however, if we consider tooth extractions to be low- or standard-risk surgery, then anticoagulation should be stopped one day before the procedure.
An alternative, theoretical approach is to perform the procedure when plasma levels of the NOAC are at a trough — this approach could be especially suitable for patients who are at a high risk of stroke.
Such patients should arrange to have their procedure first thing in the morning and:
- For apixaban and dabigatran, omit the morning dose and recommence with the evening dose if bleeding has resolved
- For rivaroxaban, withhold the dose that morning and take it later in the day (within 12 hours) if bleeding has resolved
Similar advice would apply for patients having cataract removals.
Adverse effects
Like for warfarin, the main adverse effect of NOACs is bleeding — clinicians should make clear to patients that these new medicines are not without the risk of haemorrhage.2 The details of this communication should be documented clearly in the patient notes.
There is no licensed antidote to reverse the effects of NOACs as there is for warfarin. Signs of internal or external bleeding include:
- Bruising
- Epistaxis
- Haematuria
- Haemoptysis
- Haematemesis
- Malaena
- Abnormal genitourinary bleeding
Patients should report any minor recurrent bleeding or malaena to their GP immediately. They should also seek emergency care if they suffer a blow to the head, are involved in a major trauma or suffer profuse or prolonged bleeding (more than 15 minutes).
Patients who frequently had INRs above the therapeutic range when they were taking warfarin may notice that their tendency to bleed or bruise is lower after switching to a NOAC.
Further information on side effects for the three NOACs is set out in Box 3.

References
- Chowdhury R, Khan H, Heydon E, et al. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. European Heart Journal 2013;34:2940.
- Medicines and Healthcare products Regulatory Agency. Drug Safety Update. October 2013. www.mhra.gov.uk (accessed 22 October 2013).
NOTE
Clinical Pharmacist PRACTICE TOOLS do not constitute formal practice guidance. Articles in the series have been commissioned from independent authors who have summarised useful clinical skills.
You might also be interested in…
