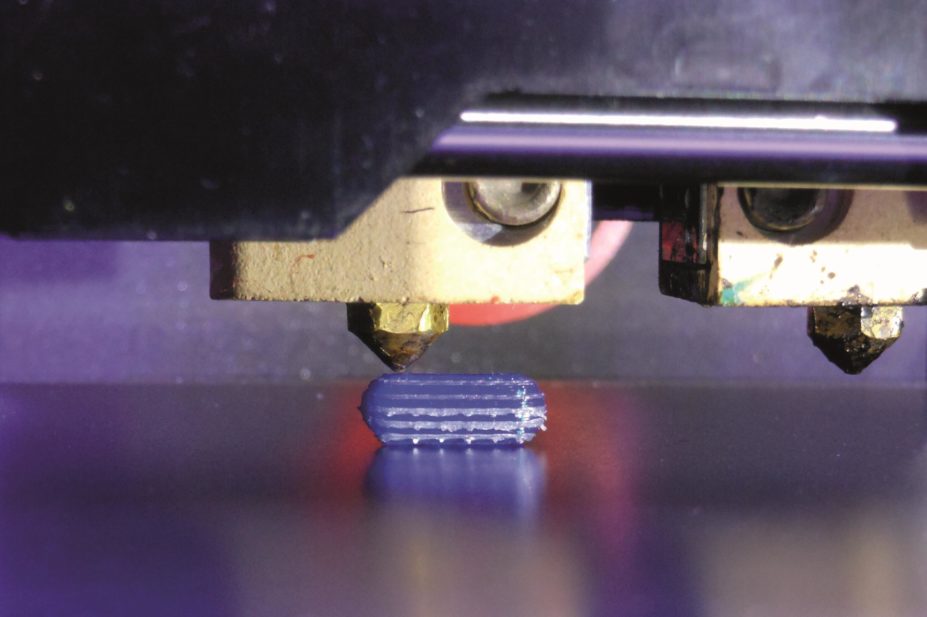
Alvaro Goyanes
Imagine a paediatrician talking to a four-year-old child who is having trouble adjusting to taking daily doses of steroids after being diagnosed with Duchenne muscular dystrophy the previous month. “What’s your favourite animal?” she asks. “A zebra,” quietly replies the child, who we will call Sam. The paediatrician smiles as she makes a note on her office computer. “But not a black and white one, a blue and green one,” adds Sam, with a little more confidence. Later, the toddler watches with wide eyes as the uniquely coloured, zebra-like tablets appear from a three-dimensional (3D) printer in the hospital pharmacy.
This story may sound far-fetched, but 3D printing promises a future of drugs printed on demand, to custom doses, and the possibility that cost may no longer be a barrier to making niche medicines. And children could be among the patients to benefit most.
“This technology could revolutionise the way we look at children’s medicines, both in terms of what they take and the ability to keep changing the dose as they grow,” says Steve Tomlin, consultant pharmacist at Evelina London Children’s Hospital, UK. Having a 3D printer in a hospital pharmacy could make weekly medication changes simple, personalised, and even fun.
Tomlin explains that the enormous weight range among children — from about 0.5kg to 100kg — means that they all need different doses of their medicines. “Currently it is impossible to have a tablet on the market to suit every size of child. This is why we use liquid medicines for children,” he says. The popular belief that children will not or should not swallow pills is false, Tomlin adds. “Studies show that most four-year-olds would actually rather take tablets.”
A 3D printer works by adding materials layer by layer until a 3D shape emerges. So far, different ‘inks’ have been used to print everything from pizza to heart valves. If a 3D printer ink that is laced with a drug can be developed, then why not print tablets as well?
This is an idea that has caught the imagination of a number of academics and pharmaceutical companies, who are aiming to develop not just the technology but the quality control needed to bring 3D printing to pharmacies.
The potential of 3D printing is about being able to deliver what you want when you want
“The potential of 3D printing is about being able to deliver what you want when you want,” says engineer Ricky Wildman from University of Nottingham in the UK. Wildman is trying to find the right materials that can be used as inks to make tablets with varying doses of drugs. In particular, Wildman is looking at inkjet 3D printing. Picture that familiar clunky printer that sits in your office or study and squirts out different coloured inks to print your photographs. Well Wildman has replaced coloured inks with polymers, drugs and other materials used in pill manufacture. The tablet is then printed layer by layer, by squirting out these ingredients into the desired shape and letting them set.
Wildman is looking closely at the materials he prints with. “In inkjet we’re exploring the way you can create suspensions and liquid-based materials that can be triggered to make solids,” he says. But he acknowledges that real-world applications are some years off, perhaps 5, 10 or even 15 years hence.
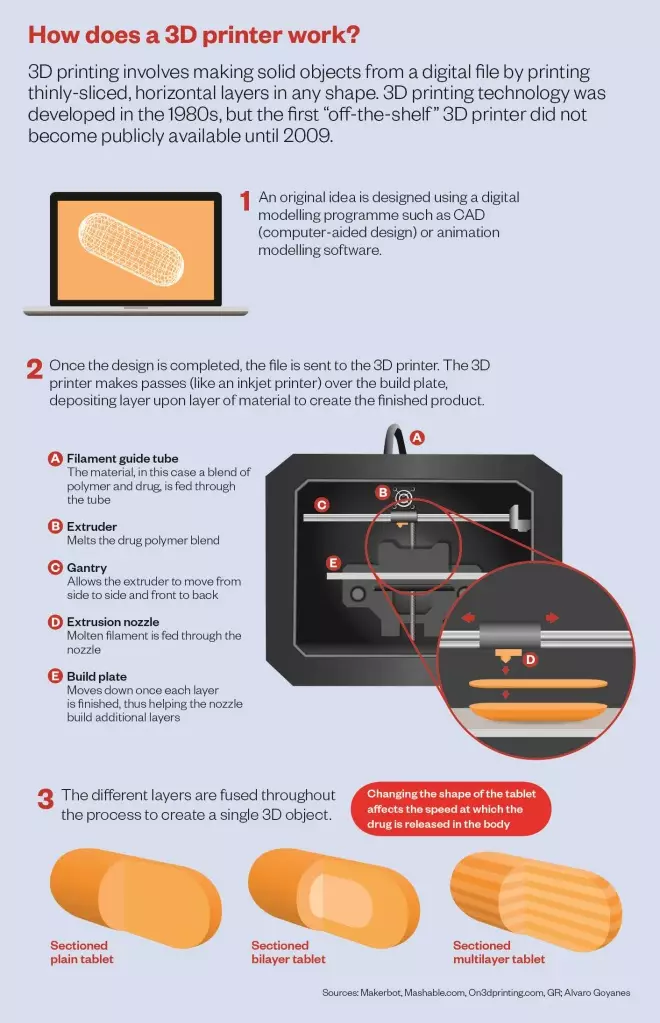
Different techniques
Other groups are also looking at applying 3D printing to drug manufacture. Simon Gaisford, a pharmaceutical scientist at University College London, is combining 3D printing and hot-melt extrusion (HME) — a technique already used in the pharmaceutical industry to make polymer blends of drugs that are not very soluble. When HME is used, a drug and polymer are heated, mixed and squeezed through a small aperture. This disperses the drug in the polymer, and this polymer can then be shaped into a tablet.
By combining the ability, conferred by HME, to work with drugs that are not very soluble with the possibility of making bespoke shapes using 3D printing, Gaisford hopes to manufacture a variety of drugs with different doses and configurations.
Eventually, he sees this technology developing so that pharmacists can tailor-make drugs for each patient. Polymers are already used by pharmaceutical companies, and Gaisford suggests that in the future pharmacists could purchase polymer inks pre-loaded with a drug and then print out pills at a local dispensary. This approach of ‘mixing up’ medicines for each individual patient echoes the way drugs used to be dispensed many years ago.
So far, Gaisford and his team have tested their printing prowess on two aminosalicylate drugs used to treat inflammatory bowel disease[1]
. They applied a process related to HME called fused-deposition modelling, in which a heated polymer is squeezed out of the printer tip and then solidifies. They printed tablets in a range of shapes and found that the different shapes affected the speed at which the drug was released in the body — for example, a pill with a hollow middle dissolved at a different rate to one in which more of the middle was filled in.
The trouble with this technology is finding the right materials, says Mohamed Albed Alhnan, a pharmaceutical scientist at the University of Central Lancashire in Preston, UK, who is looking at a similar approach. So far Alhnan has printed the steroid prednisolone in differing doses[2]
, and an anti-asthmatic drug theophylline.
Any polymer used in drug manufacture needs to be biocompatible but also able to withstand the high temperatures used during the printing process, Alhnan says. He has had some success in finding polymers that can be processed at high temperatures, although still lower than the typical 220–255 °C used in non-pharmaceutical 3D printing applications. With a patent pending on the technology and a paper on the way, Alhnan is reluctant to say more.
Democratising pharmaceuticals
3D printing is often considered to be at the forefront of the ‘democratisation of technology’ — the idea that technology can bring anything to anyone. From suggestions that in the future you will be able to print off your own T-shirt without having to resort to a sweatshop-produced version, to the hope that a farmer in a remote field in India who has a broken tractor can go to his local 3D print shop and get a new cambelt, the promise seems endless.
For Lee Cronin, a chemist at the University of Glasgow in Scotland, the developing world is where 3D drug-printing would be most useful. “Personalisation is the ‘sexy’ driver but I think distribution and reach are the winners here — especially in the developing world,” Cronin says. He has his own take on 3D printing — he is developing a system called reactionware, in which a 3D printer prints out the necessary kit to perform the entire synthesis of any molecule.
Personalisation is the ‘sexy’ driver but I think distribution and reach are the winners here — especially in the developing world
“I would get away from 3D printing as a concept and more look at the 3D printer as a cheap configurable chemical and formulation robot,” he says. In Cronin’s reactionware, the chemical starting materials are printed, as is the equipment needed to mix, transfer, analyse and purify the molecule. “We are making big strides in combining chemical synthesis, purification and control within the devices,” Cronin says. Without the need for specialised equipment (the printer does everything), reactionware has the potential to enable poor and remote communities to manufacture any drug they need.
Wildman is also excited about the idea of using 3D printing to increase access to medicines. “You could create mini factories distributed and set up for the most frequent type of drugs used,” he says. In remote locations, the ‘factory’ would be the local pharmacy, he suggests.
Industrial interest
But getting 3D drug-printing off the ground first requires some interest from industrial partners. This could take time: “The pharmaceutical industry is a conservative one,” says Wildman. There is some progress in this area, though. Aprecia Pharmaceuticals, based in Langhorne, Pennsylvania, filed its first 3D-printed product for approval to the US Food and Drug Administration (FDA) in October 2014. The company is developing a system that can print large doses of drugs in a formulation that makes them easy to swallow. Aprecia’s product, called ZipDose, is built up from layer upon layer of powders of the drug bound together by droplets of liquid. When the pill is taken with a sip of water it disintegrates very quickly, which makes taking high doses a breeze, Aprecia claims.
Jennifer Zieverink, Aprecia’s senior director of alliance management, explains that the key drivers for using this method of manufacture are the ability to be more precise and to make drugs more patient-friendly. “We hope to ultimately improve adherence by alleviating medication avoidance issues related to hard-to-swallow or hard-to-administer dosage forms,” she says.
The company has invested in facilities, and is gearing up to create 150 jobs in its manufacturing plant in Blue Ash, Ohio.
Elsewhere, Gaisford has started a company based in Ashford, UK, to commercialise his technology, called FabRx. And pharmaceutical giant GlaxoSmithKline (GSK) is running a research and development project looking at 3D printing of drugs in its Upper Merion, Pennsylvania, site. The project is at an early stage and is more about assessing whether GSK should be investing in 3D printing of drugs than doing it for now. Firstly, GSK wants to determine whether there are certain types of drugs that might benefit from being manufactured using this method, and, if so, what materials and systems they might need to start a 3D printing programme.

Source: Niklas Sandler
Niklas Sandler is investigating hyperspectral imaging as a way of building quality control into printing drugs
Quality control
Of course, any commercial effort to produce drugs using 3D printing will have to comply with regulations, an issue that will require some serious thought. “One wouldn’t want to place a pharmaceuticals factory in someone’s home that wasn’t regulated,” says Wildman. Alhnan speculates that it may make sense to regulate the finished, printed product but suggests that printed medicines would also need to be manufactured under the supervision of someone with a licence to operate a 3D printer to dispense drugs.
And for regulators to be confident in any licences they grant, they need to know that the printers will give the same product each time. This will require validation, says Wildman. “The challenge is to ensure that you are creating what you said you were going to create,” he says. To do this, a portfolio of safe materials that can be regulated and used as standard will need to be established.
Niklas Sandler, professor in pharmaceutical technology at Ã…bo Akademi University in Turku, Finland, is focusing his 3D printing efforts on building quality control into the process. Sandler is playing around with lots of proof-of-concept ideas for printing drugs with different doses, layer by layer. To check that each layer contains the correct amount of drug, Sandler is looking at a technique called hyperspectral imaging. This method takes tens of thousands of spectral images at one time across an entire sample, with each spectrum becoming a pixel in an overall image of what the sample contains, chemically, at each point. Sandler has shown that the technique works in inkjet 3D printing of drugs, using theophylline as a model compound[3]
. He is confident that hyperspectral imaging can be integrated into the printing process in the future. “When prices go down, it will be inexpensive,” he adds.
As well as clinicians being able to control doses accurately, regulators should also benefit from this kind of in-line monitoring, says Sandler. “You can build in the quality in your system if you’re able to monitor every single thing,” he says. High quality control should give regulators the confidence that patients will get what it says on the tin.
So will 3D printing completely replace drug manufacturing as we know it? Or is it more likely that 3D printing’s future lies alongside traditional ways of making pills? It depends who you talk to. “You wouldn’t want to make ibuprofen with a 3D printer,” says Wildman, who thinks the technology will be used for niche drugs that might not be manufactured otherwise, even if they had been discovered and tested in the clinic. Cronin is more ambitious for the technology: “In the end it will replace big plant [drug manufacturing] all together. That’s my vision, anyway.”
References
[1] Goyanes A, Buanz ABM, Hatton GB et al. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. European Journal of Pharmaceutics and Biopharmaceutics 2015;89:157–162.
[2] Skowyra J, Pietrzak K & Alhnan MA. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. European Journal of Pharmaceutical Sciences 2015;68:11–17.
[3] Vakili H, Kolakovic R, Genina N et al. Hyperspectral imaging in quality control of inkjet printed personalised dosage forms. International Journal of Pharmaceutics 2015;483:244–249.
You may also be interested in
Competition watchdog launches investigation into discontinuation of bipolar drug

Warwick Smith: ‘The pandemic has removed factors that allowed us to be resilient for Brexit’
