Shutterstock.com / The Pharmaceutical Journal
Alain Fischer first saw the potential of gene therapy in an unexpected way. In the early 1990s, the paediatric immunologist at the Necker Hospital for Sick Children in Paris was treating children born with severe combined immunodeficiency (SCID), a group of genetic disorders that leave children without a functioning immune system. Such children are extremely vulnerable to infections and are often unable to live a normal lifestyle. But every once in a while, one of Fischer’s patients became able to fight off many normal infections out of the blue.
Fischer and his colleagues soon worked out that these patients improved because a type of immature immune cell in their bone marrow developed a genetic mutation — one that, against all odds, corrected the genetic defect that had caused the immunodeficiency in the first place. “We thought that actually what happened in these patients was a kind of natural gene therapy,” Fischer recalls.
For a quarter of a century, Fischer and scores of other researchers around the world have been trying to replicate this rare feat by designing their own approaches to gene therapy, a method of treating disease by introducing new genetic material into a patient’s cells.

Source: Robin Ali / UCL Eye Therapy
Robin Ali, a geneticist at University College London, is working on gene therapies for a variety of eye diseases
Their efforts might soon pay off. “Gene therapy is really at the point of clinical application,” says Robin Ali, a geneticist at University College London (UCL) who is working on gene therapies for a variety of eye diseases.
Although a commercial gene therapy for cancer was approved in China in 2004, regulators in the United States and Europe have been more sceptical of the technology, with the US yet to approve a gene therapy treatment. The first gene therapy to be licensed in Europe, called Glybera (alipogene tiparvovec), was approved in November 2012 and is expected to roll out by early 2015. Marketed by the Amsterdam-based biotech company uniQure, it treats a rare metabolic disorder called lipoprotein lipase deficiency, in which individuals lack the ability to break down fatty acids in the blood, leading to inflammation of the pancreas that is painful and sometimes fatal.
The drug’s approval has been controversial, with some saying that it got the go-ahead simply because clinical trials demonstrated its safety, not because they showed effectiveness. “I’m not impressed by these data, and people have been surprised that this product has been marketed,” says Fischer.
Still, many researchers working on gene therapy see the development as good news. “The fact that a gene therapy was approved is a huge vote of confidence in the field,” says Jean Bennett, professor of ophthalmology at the University of Pennsylvania, who also works on ocular gene therapies.
Five to ten years ago, many people would probably have said that gene therapy has no future
The vote of confidence was especially welcome because gene therapy has had dramatic ups and downs since 1990, when the treatment was first used in humans. The four-year-old patient, who had a form of SCID caused by adenosine deaminase (ADA) deficiency, was successfully treated — but only temporarily. And in subsequent years, the deaths of two patients in other gene therapy trials — one from a massive immune reaction in 1999 and the other from leukaemia in 2003 triggered by the treatment — led many to write-off gene therapy altogether.
“Five to ten years ago, many people would probably have said that gene therapy has no future,” Fischer says.
No single study has been a watershed and turned the field around. But over the past few years, a steady accumulation of results from clinical trials of gene therapy for perhaps half a dozen different diseases has spurred many more researchers to enter the field.
“There’s a huge amount of interest at the moment from pharma and from venture capital about developing this area,” adds Ali, who is currently in negotiations to form a spin-off company to commercialise some of the gene therapy approaches developed in his lab at UCL.
As well as spin-off companies from universities, existing pharmaceutical companies are licensing gene therapy technologies and new biotech companies are springing up to develop their own approaches. According to Forbes magazine, investment in gene therapy research by US companies alone totalled over US$600m between January 2013 and April 2014.
Vector refinement
The most popular approach to gene therapy involves using a genetically engineered virus, or vector, to deliver the desired DNA into a patient’s cells. The engineered retroviruses used in the trials for SCID sometimes triggered leukaemia because they could integrate into patient’s genomes in a way that activated cancer-causing oncogenes.
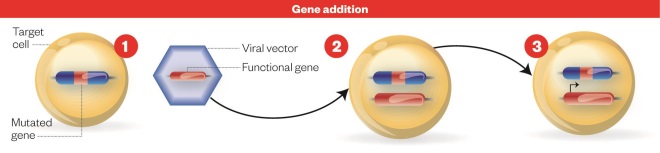
Gene addition process
Source: Nature
Gene addition: 1) Cell with defective gene. 2) New genetic material encoding a functional protein is carried to the patient’s cell using a genetically engineered virus, or vector. 3) Cell with functioning gene: the functional gene integrates with the host DNA resulting in production of the functional protein.
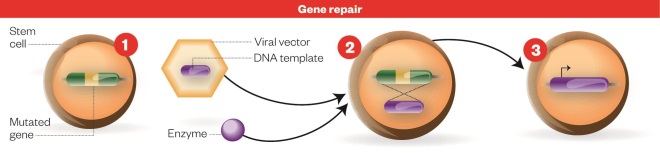
Gene repair process
Source: Nature
Gene repair process: 1) Stem cell with defective gene. 2) An enzyme that precisely edits DNA and a DNA template for the functional gene sequence are added to the host cell using a viral vector. 3) Cell with functioning gene: The mutated sequence is then replaced with the functional copy to repair the defective gene, resulting in production of the functional protein.
To prevent this, researchers have developed vectors lacking the viral regulatory sequences that could turn on oncogenes. Over the past seven years, Fischer and his colleagues have treated about 30 patients with SCID and related disorders using these so-called self-inactivating vectors, yielding effective treatment of these disorders without any cases of leukaemia, he reports[1]
.
Curiously, gene therapy for ADA-SCID has never resulted in leukaemia, even using first-generation retroviral vectors. Scientists aren’t sure why, though the reasons may have to do with the differences in the function of genes involved in different forms of SCID, or the relatively slow proliferation of immune cells that occurs after gene therapy for ADA-SCID.
GlaxoSmithKline is commercialising a gene therapy product for ADA-SCID, based on research at the San Raffaele Telethon Institute for Gene Therapy (TIGET) in Milan, Italy, which could become the first clinically approved gene therapy for any form of SCID.
Other gene therapy approaches use a vector known as adeno-associated virus (AAV), which has become popular because of its good safety profile. The DNA it carries reliably becomes integrated into the host genome at a specific location on chromosome 19, so there is less risk of gene therapy causing cancer.
Researchers say that several AAV-based gene therapies to correct haemophilia, a bleeding disorder caused by a lack of the clotting proteins Factor VIII or Factor IX, are particularly promising. In one ongoing study[2]
, five out of ten patients achieved “mild” disease, defined as bleeding only after injury and having at least 5% of normal blood levels of Factor IX. While further refinements are needed, “I’m confident now that [gene therapy will] be the treatment of choice within ten years” for haemophilia, says study team member Edward Tuddenham, a haematologist at UCL.
Although 5% of normal doesn’t sound like much, haemophilia has been an attractive target for gene therapy precisely because only a few cells need to incorporate the new DNA in order for treatment to be clinically successful. “Only a tiny amount is needed to make a very big difference to the clinical bleeding tendency,” Tuddenham says.
Tuddenham and his colleagues have also worked out how to minimise the danger from immune reactions to the gene therapy, closely monitoring patients and administering courses of immune-suppressing steroids at the first sign of trouble.
The fact is we have superbly effective treatments for mice that just about work in humans
But researchers will need to find ways to make the gene vectors more efficient at delivering DNA if they want to treat other, more common, diseases of the blood formation system, such as sickle-cell disease. Even the best AAV vectors are “rather poor at entering human cells compared to mouse cells”, Tuddenham says. “The fact is we have superbly effective treatments for mice that just about work in humans.”
Eye disease
Gene therapies that are furthest along towards clinical use tend to have several of the following factors in common: they aim to correct single-gene defects by adding a gene for a functional protein; they involve transforming cells that are easily accessible; and they only need to affect a relatively small number of cells in order to be successful. Haemophilia clearly fits that profile. So do many inherited causes of blindness.
“The eye is a good organ to develop novel therapies,” says Ali. The eye is small and accessible, so scientists can deliver a gene therapy reagent precisely to the retina, the layer of light-sensitive cells at the back of the eye, and easily monitor the health of the structure afterwards. In addition, there is little activity of immune cells inside the eye, so relatively low risk of a harmful immune reaction to the gene therapy.
The first ocular gene therapy to reach the market may be one aimed at correcting a defect in a gene called RPE65, which causes one form of inherited vision loss called Leber’s Congenital Amaurosis. Bennett and Ali are both part of teams working independently on RPE65 gene therapy[3],[4]
, which is now in phase II and III clinical trials.
But that’s merely the tip of the iceberg: over 150 inherited single-gene defects are known to cause retinal degeneration. “There are lots and lots of eye conditions that could be amenable” to gene therapy, Ali says.
In fact, the sheer number of eye conditions poses a problem because it would be impractical and far too expensive to develop specific gene therapy for each individual disorder. For this reason, Bennett and her colleagues at University of Pennsylvania are also working on an approach to deliver a gene that encodes a protein to help keep retinal cells healthy for longer, potentially stalling a host of retinal degenerative diseases.
Another broad approach is ‘suppression/replacement’ gene therapy that could treat many disorders involving rhodopsin, a light-sensitive pigment in the eye. This two-step approach knocks out a patient’s own rhodopsin genes and introduces a new, functional gene in its place. “It’s a mutation independent strategy. And I think it’s quite clever,” Ali says. But it’s also risky: a researcher has to be absolutely confident of that second “replacement” step, or else gene therapy could make a patient’s vision much worse.
Chimeric antigen receptor technology
The uptick of interest in gene therapy is also inspiring researchers to think beyond hereditary diseases involving single genes to develop treatments for acquired disorders involving the interaction of multiple genes and environmental factors.
Some of the most advanced work in this area is an approach to cancer treatment in which immune cells called T cells are removed from a patient’s bloodstream, given a gene encoding protein called a chimeric antigen receptor that targets tumour cells, before being reinfused back into the patient’s bloodstream.

Source: Bruce Levine / University of Pennsylvania
Bruce Levine, director of the clinical cell and vaccine production facility at the University of Pennsylvania, is developing gene therapy for HIV infection
In 2011, researchers reported that of three patients with chronic lymphocytic leukemia treated with gene therapy, one had a 95% reduction in tumour burden and the other two had their cancer cells disappear entirely[5],[6]
. These latter two remain cancer-free four years after receiving the treatment, says study team member Bruce Levine, director of the clinical cell and vaccine production facility at the University of Pennsylvania.
Though the study was small, its dramatic results prompted an explosion of interest in chimeric antigen receptor (CAR) technology. One of the strengths of the approach is that it is adaptable to many different cancers, by changing one component of the CAR gene to correspond to molecules expressed on the surface of a particular type of cancer cells.
More than 100 patients have received treatment based on this principle at the University of Pennsylvania, most with leukaemias and lymphomas but also including some patients with mesothelioma, pancreatic cancer and breast cancer. In August 2012, the team at the University of Pennsylvania signed an agreement with Novartis to commercialise the technology. And at least half a dozen more centres around the world are now running trials of similar cancer gene therapies.
Blocking HIV entry
Modified T cells are also central to gene therapy treatment for HIV infection. This approach grew out of observations that about 1% of people of European descent lack functional CCR5, a cell-surface receptor that HIV needs in order to enter the T cells in which the virus replicates. So the researchers set out to use gene therapy to create T cells that HIV is unable to enter.
Researchers collect T cells and insert into them a gene for a zinc-finger nuclease, a specially designed enzyme created by Sangamo BioSciences that cuts precise sequences of DNA. As the T cells proliferate, the zinc-finger nuclease alters the CCR5 gene to produce the HIV-resistant variant, which is known as delta32.
In April 2014, Levine and his collaborators reported that among six HIV-positive patients who received this therapy and then temporarily stopped taking antiretroviral therapy, the gene therapy showed anti-HIV effect in four of them[7]
.
In one patient, in fact, the virus disappeared completely, even when he was not taking antiretroviral therapy. The researchers found that he carried one copy of the delta32 form of CCR5 in his own genome. “This guy had a 50-yard start in the 100-yard dash,” Levine says — that is, he already had some T cells that were resistant to HIV even before the therapy was administered.
In order to achieve a similarly dramatic result in more patients, the researchers will have to increase the efficiency of gene transfer to the T cells, as well as the proportion of infused cells that take-up residence in the bone marrow. Levine’s group is working with Sangamo on several strategies to achieve this, perhaps including delivering DNA directly to cells by electroporation — saps of electricity create brief openings in the cell membrane, enabling the DNA molecules to enter — rather than using a viral vector.
Gene correction
Along with efforts to develop new applications of gene therapy come attempts to imagine new ways of editing the genome. In early 2014, researchers in Italy reported the first use in a human patient of technology that precisely edits DNA to repair a defective copy of a patient’s gene[8]
. In this case, the researchers used a zinc-finger nuclease similar to those that have been used to disrupt the CCR5 gene in HIV patients. Gene therapy researchers are also working to apply other precise gene editing technologies such as TALENs (transcription activator-like effector nucleases) and CRISPR (clustered regularly interspaced short palindromic repeats) to the field.
Fischer, who is not involved in the work, describes this gene correction approach as “more elegant but more difficult to implement” compared to the standard gene addition method of gene therapy. That’s in part because cells can only incorporate the repaired DNA when they are in the process of dividing. “It’s easier to say than it is to make,” says Fischer, “but it is feasible.”
Elegant, and easier said than done, yet increasingly achievable: he may as well be describing gene therapy in general.
References
[1] Fischer A, Hacein-Bey-Abina S, Cavazzana-Calvo M. Gene therapy of primary T cell immunodeficiencies. Gene 2013;525:170–173.
[2] Nathwani AC, Tuddenham EGD, Rangarajan S et al. Adenovirus-Associated Virus Vector–Mediated Gene Transfer in Hemophilia B. New England Journal of Medicine 2011;365:2357–2365.
[3] Testa F, Maguire AM, Rossi S et al. Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital Amaurosis type 2. Ophthalmology 2013;120:1283–1291.
[4] Bainbridge JW, Smith AJ, Barker SS et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. New England Journal of Medicine 2008;358:2231–2239.
[5] Porter DL, Levine BL, Kalos M et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. New England Journal of Medicine 2011;365:725–733.
[6] Kalos M, Levine BL, Porter DL et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Science Translational Medicine 2011;3:95ra73.
[7] Tebas P, Stein D, Tang WW et al. Gene Editing of CCR5 in Autologous CD4 T Cells of Persons Infected with HIV. New England Journal of Medicine 2014;370:901–910.
[8] Genovese P, Schiroli G, Escobar G et al. Targeted genome editing in human repopulating haematopoietic stem cells. Nature 2014;510:235–240.