Eye of Science / Science Photo Library
Lung cancer is the leading cause of cancer-related deaths worldwide, with only 9% of patients in the UK surviving for at least 5 years after diagnosis, a statistic that has shown little improvement over the past 40 years. But a new form of immunotherapy — based on inhibition of programmed death-1 (PD-1) receptors and programmed death-ligand 1 (PD-L1) — is showing promise in some lung cancer patients and offers patients and clinicians new hope.
“PD-1/PD-L1 inhibitors look important across a pretty wide swathe of cancers. What got people excited was a lesser [compared with melanoma] but still significant response rate in non-small-cell lung cancer (NSCLC), as well as in bladder, and head and neck cancers,” says Louis Weiner, an immunotherapy expert and gastrointestinal oncologist at Georgetown University in Washington, DC. While melanoma has typically been viewed as immunoresponsive, lung cancer has been thought to be unresponsive to immunotherapy, so the response rate seen with PD-1/PD-L1 inhibitors has clued the oncology world on to the fact that this new generation of immunotherapy might be different.
Many experts say that out of all of the therapies developed to harness the power of the immune system to fight cancer over the past several decades, PD-1/PD-L1 inhibitors look to be the most promising, even though it is still relatively early days in their development.
Our real hope is that we can take a terminal disease and for some, at least for now, provide them with long-term survival
While differentiating themselves from other immunotherapies in terms of the numbers of cancer patients who respond to them across a number of different tumour types, the PD-1/PD-L1 inhibitors also appear to offer an advantage compared with standard therapy in terms of their duration of response, although so far follow-up of trials has been at most a few years. “Our real hope is that we can take a terminal disease and for some, at least for now, provide them with long-term survival,” says Dan Chen, who heads up the PD-L1 programme at biotechnology company Genentech, a subsidiary of Roche. While some of the newer targeted therapies and traditional chemotherapy drugs that dominate the oncology drug market produce higher response rates than PD-1/PD-L1 inhibitors, they commonly result in response durations measured in months rather than years, because resistance almost always develops to traditional drugs among initial responders.
“Cancer immunotherapy has offered an idea and a hope for a hundred years, but you couldn’t go to your doctor’s office and get a successful immunotherapy,” says immunologist Gordon Freeman of Dana-Farber Cancer Institute in Boston, Massachusetts, who was one of the first to discover the PD-L1 protein and elucidate its function in shutting down the T-cell response against cancer. “This began to change with interleukin-2 (IL-2) therapy, [Dendreon’s therapeutic cancer vaccine] Provenge, and anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) therapy, but all have their weaknesses.”
In September 2014, the US Food and Drug Administration (FDA), granted accelerated approval to the PD-1 receptor inhibitor Keytruda (pembrolizumab), developed by Merck, headquatered in New Jersey, to treat patients with advanced melanoma who are no longer responding to other drugs. This development looks to be just the beginning of this promising new immunotherapy across a wide range of cancers.
Bristol-Myers Squibb (New York), which since 2011 has marketed the anti-CTLA-4 monoclonal antibody Yervoy (ipilimumab) — the first approved immune checkpoint inhibitor — for advanced melanoma, has filed its fully human PD-1 inhibitor Opdivo (nivolumab) with the FDA and the European Medicines Agency (EMA) for previously treated advanced melanoma. The drug secured Japanese approval in July 2014 through partner Ono Pharmaceuticals (Osaka, Japan). Bristol-Myers Squibb has also completed its filing for pre-treated squamous NSCLC with the EMA and has initiated a rolling submission in the United States.
Meanwhile, Roche (Genentech; California) has reached phase 3 development with its PD-L1 inhibitor, MPDL3280A; AstraZeneca and its global biologics R&D arm MedImmune (Maryland) are also in phase 3 development with their PD-L1 inhibitor MEDI4736, and earlier clinical development with their PD-1 inhibitor MEDI0680 (AMP-514); and Merck Serono, the biopharmaceutical division of Merck KGaA (Darmstadt, Germany), is conducting a phase 2 trial with its PD-L1 inhibitor MSB0010718C.
Giving a boost to the belief that this class of drugs has promise across a number of different tumour types, the FDA has granted Keytruda “breakthrough therapy” status for previously treated metastatic melanoma and previously treated NSCLC; Opdivo for previously treated melanoma and previously treated Hodgkin’s lymphoma; and MPDL3280A for metastatic bladder cancer.
The pathway
Both PD-1 and PD-L1 inhibitors target the interaction between PD-1 and PD-L1. The PD-1 receptor is expressed on the immune system’s T cells and B cells, while its ligand PD-L1 is expressed on a variety of cells in the body, including many tumour cells. The interaction between PD-L1 and PD-1 results in dampening an immune response.
The story of PD-1’s discovery began in the early 1990s, when immunologist Tasuku Honjo of Kyoto University School of Medicine identified a gene that was upregulated during T-cell activation and cell death. They named this gene programmed death-1 (PD-1). Clues to its biological function and its potential to be exploited in cancer immunotherapy were then elucidated over the next decade.
In 1999, Honjo discovered that mice lacking the gene developed a lupus-like syndrome, suggesting that PD-1 is involved in downregulating the immune response[1]
. Freeman and his colleague Arlene Sharpe, an immunologist at Harvard Medical School, discovered the natural ligands for the PD-1 receptor — called PD-L1 and PD-L2 in 2000 and 2001 — showed they inhibited T cell responses, and found that they were expressed by some cancer cell lines.
Immunologist Lieping Chen, now of Yale University School of Medicine, showed in 2002 that multiple tumour types express PD-L1 and that it can cause cell death of tumour specific T cells. In 2002 and 2003, Honjo and Chen showed that antibodies against PD-L1 could lead to tumour rejection.
PD-1/PD-L1 inhibitors work differently than most cancer immunotherapy strategies tested in the clinic, which have typically been aimed at stimulating a T-cell response against specific tumour antigens. One reason these prior approaches, including therapeutic cancer vaccines, have not generally been successful is that T-cells have “checkpoints” like PD-1 and CTLA-4, to guard against autoimmunity and to protect tissues from damage by an over-exuberant immune response to an infection. The tumour can also resist such immunostimulatory approaches by taking advantage of these natural protective, inhibitory mechanisms.
“When you stimulate an immune response, T-cells make interferon which promotes PD-L1 expression by the tumour cells and this shuts down the T-cell response against the tumour. When you stimulate, you also put on the brakes,” says Freeman.
“The limiting step is not to turn on the immune system against cancer, but to avoid getting turned off by immune checkpoints,” adds Antoni Ribas, a melanoma specialist at the University of California, Los Angeles.
The first proof-of-principle for immune checkpoint inhibitors came from the anti-CTLA-4 therapy ipilimumab in metastatic melanoma, where it leads to a response in about 10–15% of patients. Although it improved median overall survival only by some months, among many of those who initially responded, survival has been years long.
While both are “checkpoint inhibitors,” anti-CTLA-4 and PD-1/PD-L1 inhibitors appear to work at different points in the immune response. PD-1 is thought to predominantly regulate effector T-cell activity within tissue and tumours, whereas CTLA-4 is thought to predominantly regulate T-cell activation in the priming phase. This may be the reason why PD-1/PD-L1 inhibitors have a significantly lower rate of high-grade, immune-related toxicities, such as colitis, compared with anti-CTLA-4 therapy.
Taking off the brakes
Tumour cells can evade the body’s immune system by turning it off just as it begins to mount a response against them. However, scientists have discovered how to block this “immune checkpoint” and let the body continue its attack.
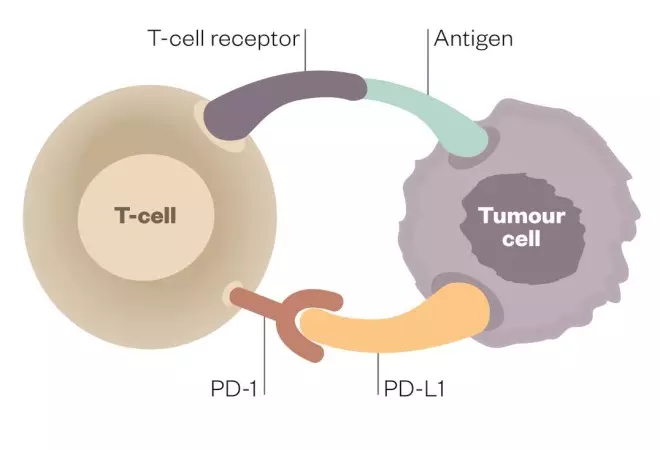
Deactivated T cell
When programmed-death receptor (PD-1) on the T cell binds to programmed death-ligand 1 (PD-L1) on the tumour cell, the T cell becomes deactivated, allowing the cancer cell to evade immune attack
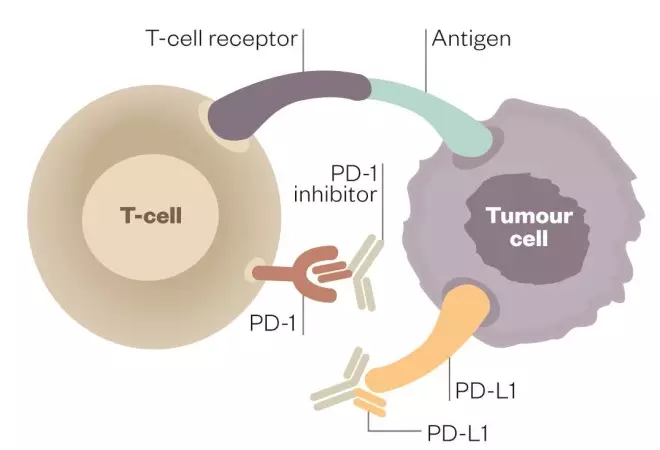
Activated T cell
Inhibitors of programmed-death receptor (PD-1) and programmed death-ligand 1 (PD-L1) can prevent the tumour cell from binding to PD-1, enabling the T cell to remain active and co-ordinate an attack
Comparisons
Compared to other previously tested immunotherapies, PD-1/PD-L1 inhibitors, when used as monotherapies, appear to shrink tumours in a much higher proportion of patients, across a wider range of tumour types and with a lower rate of high-grade toxicities, mainly immune-mediated side-effects. They are also less labour-intensive to administer than some other types of immunotherapy, not requiring personal preparation for each patient as do dendritic cell vaccines like Provenge and chimeric antigen receptor (CAR) T-cell therapy.
“PD-1 inhibitors appear to produce the highest response rate of any single-agent immunotherapy,” says Michael Postow, a melanoma specialist at Memorial Sloan-Kettering Cancer Center in New York. “They are the first to break through the ceiling of about a 10% response rate,” adds Adil Daud, a melanoma specialist at University of California, San Francisco. In melanoma, across various trials so far, between 25% and 40% of patients have responded, according to the standard RECIST criteria (a response is declared when the tumour shrinks by at least 30%). Response rates have been slightly less in renal cell carcinoma (RCC), which, like melanoma, has historically been viewed as a tumour type that has responded to immunotherapy.
PD-1 inhibitors appear to produce the highest response rate of any single-agent immunotherapy
Compared with standard therapies, including chemotherapy and “targeted” therapies, immunotherapy, including PD-1/PD-L1 inhibitors, appears to result in a longer duration of response. “The promise of cancer immunotherapy is that we see patients with a much more durable response, who are alive two, three, five, ten years on,” says Genentech’s Chen. The longer durations of survival have been seen with anti-CTLA-4 therap, for which follow-up of patients from trials has been longer than for PD-1/PD-L1 inhibitors.
“The immune system has two attributes that any targeted therapy lacks — the capacity to remember and the ability to detect and destroy resistant variants as they emerge,” explains Weiner. “Targeted therapies usually focus on one narrow target, which the cancer soon learns to evade, while immunotherapy can hit multiple targets, which is harder for the cancer to escape,” adds Freeman.
Melanoma
For PD-1 inhibitors, the data are most mature for melanoma, where it appears that the longer duration of response, coupled with a substantial proportion of responders, has produced the first overall survival benefit seen with any of these agents in a phase 3, randomised, controlled trial.
The promise of cancer immunotherapy is that we see patients with a much more durable response, who are alive two, three, five, ten years on
Bristol-Myers Squibb announced in June 2014 that it was stopping early its phase 3 study comparing PD-1 inhibitor nivolumab with dacarbazine chemotherapy in 418 patients with previously untreated, BRAF wild-type advanced melanoma after an overall survival benefit was observed. The data, just published, show that the overall survival rate at one year was 73% in the nivolumab group and 42% in the dacarbazine group; median overall survival has not yet been reached in the nivolumab group and was 11 months in the dacarbazine group[2]
. Dacarbazine is still used as a first-line therapy for metastatic melanoma in many countries, although ipilimumab has now become the current standard in others for melanoma patients without a BRAF mutation.
Most other data on PD-1/PD-L1 inhibitors reported so far have been from phase 1, non-randomised, non-controlled studies that have tested different doses and schedules of the drugs, and have not yet reported follow-up for more than three years. However, clinicians and companies say that the data in terms of duration of response, as well as one-year, two-year and in some cases three-year overall survival rates, look much better compared with standard therapy.
The new class PD-1/PD-L1 inhibitors can give durable responses in patients with metastatic, solid tumours — it is a whole new way of looking at things
For example, in a Phase Ib dose escalation study of nivolumab, 41% of melanoma patients were still alive at three years[3]
and 45% of NSCLC patients were still alive at two years at the dose being taken forward into phase 3 studies[4]
. “Those survival rates have not been seen with any therapy,” says Michael Giordano, senior vice-president and head of oncology development at Bristol-Myers Squibb.
The reason for what appears to be higher survival rates is a long duration of response among responders. “The new class PD-1/PD-L1 inhibitors can give durable responses in patients with metastatic, solid tumours — it is a whole new way of looking at things,” says Ribas. “While we haven’t followed up patients for ten years yet, in the first two years, the majority of melanoma patients who had a response initially continue to have one.”
Wide application
Durable responses have been observed in other tumour types as well, although so far fewer numbers of patients have been tested and with shorter periods of follow-up. In NSCLC, says Genentech’s Chen, “we have patients continuing to respond for over two years… this looks very different than standard therapy.” Such a response has led to a rapid expansion of clinical trials and a race for approval in NSCLC among companies.
What will really provide the proof for PD-1/PD-L1 inhibitors are randomised, controlled trials versus standard-of-care in which they improve median overall survival. Many companies are now conducting such phase 3 studies across many different tumour types, the most common of which are melanoma, NSCLC, RCC, head and neck cancer, and bladder cancer. Bristol-Myers Squibb is also conducting a phase 3 trial with nivolumab in glioblastoma, the most deadly form of brain cancer.
In some tumour types , such as NSCLC, where response rates in the overall population are somewhat lower than melanoma but still substantial, several companies are enriching the study populations with those more likely to respond. In contrast with many other immunotherapies, for which tumour biomarkers that predict how well a patient will respond to treatment have not been discovered, PD-L1 expression by the tumour appears to be a reasonable, although not perfect, predictor of response to PD-1/PD-L1 inhibitors when they are used as monotherapies. PD-L1 expression is seen in about 30% of solid tumours and also with high frequency in certain haematologic malignancies.
“It isn’t black and white; PD-L1 negative tumours can still respond, but a lower percentage respond, while not all PD-L1 positive tumours respond, but they are more likely to respond,” says Freeman.
For now, companies are testing different cut-off points for PD-L1 tumour expression in their trials and correlating this with response in an effort to select populations of patients most likely to respond. For example, across all NSCLC tumours, an overall response rate of about 21% has been seen with Keytruda, according to Alise Reicin, vice president of oncology at Merck Research Laboratories. In the 25% of PD-L1 strongly expressing tumours (in which over 50% of tumour cells express the protein), the response rate was 37%; in the 45–70% of PD-L1 weakly expressing tumours (1–49% of tumour cells express the protein), it was 17%, and in the minority of PD-L1 non-expressing tumours it was 10%. In the phase 3 NSCLC trials it is conducting with Keytruda, Merck is including only patients with PD-L1 strongly and weakly expressing tumours.
Our vision in lung cancer is to replace chemotherapy
Bristol-Myers Squibb is also including only PD-L1 biomarker-positive patients in its phase 3 first-line NSCLC study, but is planning to test a combination strategy in biomarker-negative patients, says Giordano. If the trial — which is comparing nivolumab to the decades-old standard platinum-based doublet chemotherapy — is positive, it would be paradigm-shifting, he says. “Our vision in lung cancer is to replace chemotherapy.”
In its phase 3 NSCLC and bladder cancer trials, Genentech is including all patients, but stratifying by the PD-L1 biomarker. In a phase 1 trial, the response rate was 52% in this population of previously treated bladder cancer patients, says Chen.
Combination therapy
Some tumour types have shown little response to anti-PD-1/PD-L1 monotherapy in early clinical trials, including prostate, colorectal and pancreatic cancers. For such diseases, as well as for non-responders within the group of more immunoresponsive tumour types, combination therapy may help elicit an immune response.
“PD pathway blockade is clearly important, but pathway inhibitors will be most useful in combination therapy as the immune system has multiple ways of turning itself off, and cancer cells can highjack multiple checkpoints to escape immune detection,” says Ed Bradley, head of oncology at MedImmune.
One possibility is the combination with another immune checkpoint inhibitor. In a phase 1b dose-ranging melanoma trial, when PD-1 inhibitor nivolumab was combined with the anti-CTLA-4 monoclonal antibody ipilimumab, it resulted in an unprecedented two-year survival rate of over 80%, says Giordano[5]
. In addition, adds Postow, it did not matter whether patients’ tumours were positive for the PD-L1 biomarker with the combination strategy. However, rates of high-grade toxicity were also significantly higher. A phase 3 trial is under way to validate the result, comparing both drugs as monotherapies with the combination.
Many other companies are also testing the combination of anti-PD-1/PD-L1 therapy with anti-CTLA-4 therapy, including AstraZeneca, which is testing the combination of anti PD-L1 therapy with its own anti-CTLA-4 therapy, tremelimumab, across multiple tumour types .
Another combination AstraZeneca is pursuing in trials is that of a PD-1 with a PD-L1 inhibitor. The rationale is that, while both therapies block the interaction between PD-1 and PD-L1, PD-1 inhibitors additionally block the interaction between PD-1 and PD-L2, another ligand of the receptor, while PD-L1 inhibitors additionally block the interaction between PD-L1 and B7.1 (also a ligand for CTLA-4). Both of these additional interactions could be important to block as they can also down-modulate T-cell responses. Bradley says that in preclinical models, this combination strategy has shown synergy. However, Chen believes that blocking the PD-L1—B7.1 interaction is more important, while blocking the PD-1—PD-L2 interaction is not generally important for cancer, as there is “not a lot of PD-L2 expression in the tumour microenvironment,” but blockade could lead to increased autoimmune toxicity.
Other combination strategies include combining PD-1 pathway inhibitors with inhibitors of other immune checkpoints expressed on T-cells, such as lymphocyte-activation gene 3 (LAG3) and T-cell immunoglobulin domain and mucin domain 3 (TIM3), which have shown synergy in preclinical models, and combinations with inhibitors of immunosuppressive elements within the tumour microenvironment.
In many cases, tumours may not respond to PD-1 pathway inhibitors because the tumour antigens may not have stimulated a T-cell response to begin with. One strategy here might be to combine an immune stimulator, such as a cancer vaccine, with anti-PD-1/PD-L1 therapy. For example, in a phase 2 trial in melanoma, Amgen is testing talimogene laherparepvec (T-VEC), an investigational oncolytic immunotherapy, in combination with Keytruda.
It is also thought that standard therapies, such as radiation, targeted therapy or chemotherapy, may lead to release of tumour antigens from dead tumour cells, making the tumour more visible to the immune system. However, what is more proven is these therapies can shrink the size of the tumours, says Freeman. “If the tumour is smaller when anti-PD-1 or anti-PD-L1 therapy is administered, a benefit is more likely.” However, not all targeted therapies or chemotherapies work in synergy with immunotherapy, and each strategy will have to be proven in clinical trials; numerous such trials are ongoing.
Finally, given the belief that immunotherapy might work best when there are fewer tumour cells to eradicate, companies are also planning to test their PD-1 pathway inhibitors in the adjuvant setting, after surgical removal of localised tumours, to reduce the risk of disease recurrence. It might be here that the possibility of a “cure” is highest.
As Bradley puts it: “A good immune response can last a lifetime.”
References
[1] Nishimura H, Nose M, Hiai H et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999;11:141.
[2] Robert C, Long GV, Brady B et al. Nivolumab in previously untreated melanoma without BRAF mutation. NEJM online 16 November 2014 (accessed 17 November 2014).
[3] Hodi FS, Sznol M, Kluger HM. Long-term survival of ipilimumab-naive patients (pts) with advanced melanoma (MEL) treated with nivolumab (anti-PD-1, BMS-936558, ONO-4538) in a phase I trial. American Society of Clinical Oncology annual meeting 2014, abstract 9002 (accessed 17 November 2014).
[4] Brahmer J, Horn L, Gandhi L et al. Nivolumab (anti-PD-1, BMS-936558, ONO-4538) in patients (pts) with advanced non-small-cell lung cancer (NSCLC): survival and clinical activity by subgroup analysis. American Society of Clinical Oncology annual meeting 2014, abstract 8112. (accessed 17 November 2014).
[5] Sznol M, Kluger HM, Callahan MK et al. Survival, response duration, and activity by BRAF mutation (MT) status of nivolumab (NIVO, anti-PD-1, BMS-936558, ONO-4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma (MEL). American Society of Clinical Oncology annual meeting 2014, abstract LBA9003. Available at (accessed 17 November 2014).