Source: Carhart-Harris RL, Erritzoe D & Williams T. PNAS 2011;109:2138–2143.

Matthew Johnson who conducted a proof-of-concept trial using psilocybin to help heavy smokers quit, sits back in one of the treatment rooms at Johns Hopkins University in Baltimore, Maryland
Gordon McGlothlin is 65 years old and, until recently, he smoked 20 cigarettes a day, a habit he formed when he was just 15 years old. He tried to stop, using nicotine replacement therapy, psychological therapy or going cold turkey. But each time he relapsed. Then, McGlothlin’s friend told him about an advertisement for participants in a clinical trial of a new treatment for tobacco addiction.
So one December morning, McGlothlin walked into the research centre at Johns Hopkins University in Baltimore, Maryland, where he took a small, blue capsule and sat in a room listening to classical music. The idea was that after he walked out of the research centre in the evening he would never smoke another cigarette again. That was almost two years ago and McGlothlin says he is still smoke-free.
McGlothlin was part of a small, proof-of-concept trial using psilocybin to help heavy smokers quit[1]
. Psilocybin (pronounced silo-sie-bin) is what makes magic mushrooms psychedelic and, despite its reputation as a recreational drug for hippies, it is showing promise as a therapeutic agent for a number of psychiatric illnesses including addiction, depression and anxiety. “I think psilocybin gave me the impetus to stay abstinent. It opens up a whole new dimension to your personality. It is almost as though quitting smoking is peripheral during the experience,” says McGlothlin.
In the 1950s, 1960s and early 1970s, psychedelic drugs were extensively researched, with the effects of lysergic acid diethylamide (LSD) being studied in some 40,000 participants. But then LSD was made illegal and research ground to a halt. A retrospective analysis of six trials from the late 60s and early 70s involving 536 patients[2]
, published in 2012, found that LSD helped people overcome alcohol addiction and was “as successful as any treatment since,” says David Nutt, professor of neuropsychopharmacology at Imperial College London and a campaigner for rational drug laws that do not inhibit research. “We’re talking about one or two doses producing life-long effects.”
Psilocybin and LSD have similar, but not identical, patterns of activity in the brain. “Psilocybin hits the same primary brain receptor as LSD, called serotonin 2A,” says Matthew Johnson, associate professor of psychiatry and behavioural sciences at Johns Hopkins, who carried out the tobacco addiction trial. But, compared with LSD, psilocybin is more appealing to researchers for two reasons: its duration of action is about 6 hours compared with LSD’s 10–12 hours, which makes it more manageable to work with in a clinical setting; and, unlike LSD, it does not have the same strong association with the counterculture of the 1960s, explains Johnson. “All major drugs of abuse have accepted clinical applications, bar psychedelics. It is really exciting that these drugs could open up novel treatments,” he says, adding that “more and more” scientists are coming into the field.
Johnson recalls how the idea for his smoking cessation study came after looking at historical trials of psychedelics and noticing that their effects could be applicable to a range of addictions, since the reports of experiences were always similar. “Smoking seemed to be a good place to start.” Johnson continues: “People may say we are using a sledgehammer for smoking but it is the leading cause of preventable death worldwide and 70% of smokers in the US want to quit.”
Testing time
Johnson and his team enrolled 15 patients in the trial. “We wanted to demonstrate feasibility of the intervention,” says Johnson. On average the patients had smoked 19 cigarettes a day for 31 years and had six failed attempts to quit behind them. They all received 15 weeks of structured smoking cessation treatment, which included regular sessions of cognitive behavioural therapy (CBT) and administration of psilocybin once each at week 5 and week 7, and optionally also at week 13. It was at the first of these sessions that McGlothlin was given a blue capsule containing pure psilocybin, which takes about 20 minutes to have an effect, and told that this should be his last day smoking. After receiving the treatment, he and the other study participants would stay at the clinic for the whole day, listening to music and being encouraged by the ever-present healthcare professionals to have an introspective experience. At six months follow up, 80% of patients were not smoking.
“The results are not conclusive, but we strongly suspect that it is the psilocybin playing a role, because the quit rates are so much higher than even the best current psychological or pharmacological treatments for tobacco addiction, which are typically around 35%,” says Johnson. Based on the success of the initial trial, he and fellow researchers at Johns Hopkins have now embarked on a phase II randomised controlled trial with 80 participants, which started in October 2014.
Robert West, a health psychologist at University College London who specialises in tobacco addiction, says that the study seems to have been well thought out and conducted, and there is a plausible rationale. “While probably only a minority of smokers would be interested in using a psychedelic drug to stop smoking, these early results are worth pursuing with a comparative trial,” he says. He adds that the proposed mode of action is interesting in that it involves promoting a new outlook on life that may lead to changes in other self-destructive behaviours.
“The results are not conclusive, but we strongly suspect that it is the psilocybin playing a role.”
Evidence is mounting that this approach could tackle addiction more generally. A recent study of the psychedelic drug ibogaine found it to be effective at treating addiction to alcohol, cannabis, cocaine and crack[3]
. However, Johnson says ibogaine can have cardiovascular side effects and, in comparison, psilocybin is “very safe at a physiological level”.
Psychedelics seem to work in a different way from other treatments for addiction, such as nicotine replacement therapy, which target the same brain receptors as the drug patients are trying to wean themselves off. This difference may be central to why the treatment of addiction is just one of psilocybin’s potential uses. Recently, researchers have started to unpick its effects on brain function — with some striking results. Psilocybin decreases activity in the parts of the brain that are overactive in depression, addiction and ingrained behaviours.
Deactivating cravings
Serotonin 2A receptors, the primary target of psilocybin and other psychedelics, are located in the outer layer of the brain, the cortex. Using functional magnetic resonance imaging (fMRI), Nutt’s team found that stimulation of these receptors by psychedelics decreased rather than increased activity in certain areas of the brain, particularly those in what is known as the default mode network (DMN)[4]
. The DMN is believed to be involved in introspective thought, our sense of self and our ingrained thought patterns and behaviours. “During illnesses like depression or addiction, the default mode network in the brain becomes over-engaged with negative thoughts or cravings,” explains Nutt. When the DMN ceases to be so over-engaged it “allows people to break free” from these destructive neural patterns, he says.
Psychedelics make the brain more flexible, with this neuroplasticity potentially underlying their usefulness, says Franz Vollenweider, director at the Neuropsychopharmacology and Brain Imaging Research Unit at the University Hospital of Psychiatry in Zürich, Switzerland. But he emphasises that such agents will probably only work in conjunction with psychological therapy.
His team has discovered that the brain becomes less sensitive to negative information when under the influence of psychedelics[5]
. “In healthy volunteers we found that psilocybin reduces the processing of negative emotional stimuli. This is why we think it can be used in depression,” says Vollenweider. Part of this effect could be due to a decrease in reactivity of the amygdala after administration of psilocybin, he says. The amygdala is deep in the centre of the brain and is responsible for emotions like fear and anxiety. “Psilocybin stimulates a specific serotonin receptor which in turn induces a cascade of downstream effects.”
McGlothlin says that because of his treatment with psilocybin, he feels freed from the hold that cigarettes had over him. “It became non-important, like who cares?” He adds that the experience affected much more than just his addiction to tobacco. “Psilocybin changed my life. It’s not that I was afraid of dying, but during the experience you come to grips with the fact that life is transient and death a continuation of that process, but that your thoughts persist,” he recalls. “I had a friend dying of cancer and I think it would have been good for them, it gives tremendous piece of mind, it puts life and death in the right place, it gives you hope.” Indeed, Johnson is one of several researchers who are examining using psilocybin for exactly this purpose.

Taking a trip: How the brain is affected by psilocybin
Source: Carhart-Harris RL, Erritzoe D & Williams T. PNAS 2011;109:2138–2143.
The amygdala is the emotion centre of the brain. Psilocybin reduces amygdala reactivity to negative emotional stimuli, and has been found to induce an increase in positive mood.Neural patterns break down and the connectivity between different parts of the brain becomes more diverse and dynamic. Cognition is less constrained and perception is profoundly altered.

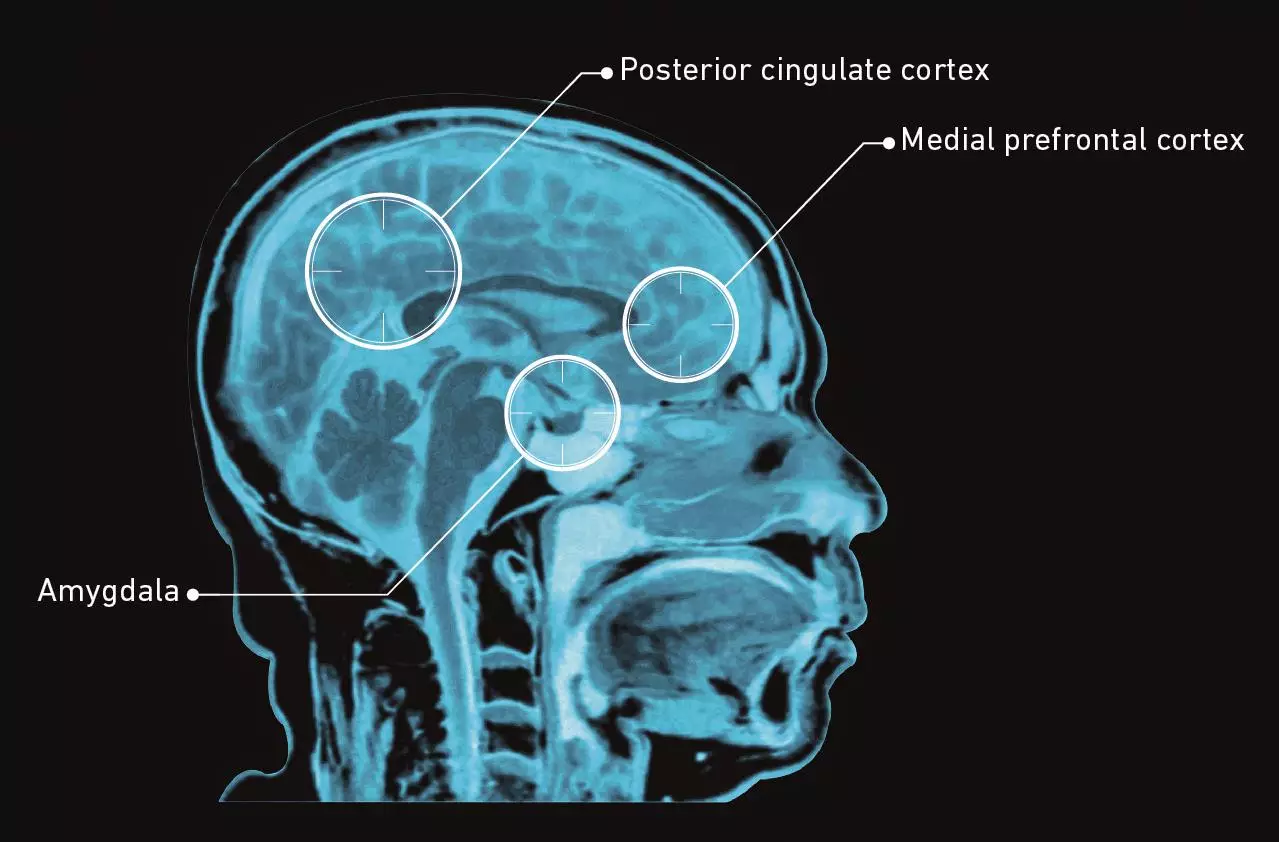
Taking a trip: Changes in connectivity of the default mode network (DMN)
Source: Carhart-Harris RL, Erritzoe D & Williams T. PNAS 2011;109:2138–2143.
The DMN is made of brain ‘hubs’, including the posterior cingulate cortex and the medial prefrontal cortex (green dot). The network is active during introspective thought, self-reflection and ingrained patterns of behaviour.In the brains of patients who have taken a placebo, the hub areas of the DMN, the medial prefrontal cortex and posterior cingulate cortex, are connected.After taking psilocybin, the hub areas of the DMN become less connected: the posterior cingulate cortex is not as strongly coupled to the medial prefrontal cortex in the brain.A decrease in DMN activity allows a less constrained mode of brain activity.
Tackling anxiety
Johnson says that work at Johns Hopkins on the use of psilocybin in cancer patients suffering from anxiety and depression is even further along than that on addiction. His team is currently analysing the results from a phase II trial of psilocybin in 44 patients with depression and anxiety about death, stemming from a diagnosis of advanced-stage cancer. Johnson says that ‘mystical’ experiences, such as McGlothlin’s, seem to lead to better clinical outcomes. The results have not yet been published, but he says that “the data are very favourable”.
In fact, in 2011, when the results of the first clinical trial to use psilocybin for over 35 years were published, it was a pilot study looking at the effect of psilocybin treatment in adults with advanced cancer and anxiety about death[6]
. The study was carried out by a separate research group at New York University (NYU). Led by Charles Grob, professor of psychiatry and paediatrics at the University of California, Los Angeles, the group found that six months after the 12 patients included in the pilot were treated with psilocybin, they reported a clinically significant improvement in mood on the Beck Depression Inventory. Although the research primarily examined anxiety, Grob says there is “a lot of co-morbidity with anxiety and depression”. The group are now conducting a full-scale trial, but their ambitions do not stop there.
“If the results from the trials at Johns Hopkins and NYU are positive, we will apply to the National Institutes for Health for a grant to conduct a larger, multi-site trial of psilocybin for anxiety and depression in patients with advanced cancer,” says Grob. And again, as Grob explains, the prompt for this direction of enquiry was research from several decades earlier, in this case identifying good treatment responses using hallucinogens for patients with terminal cancer and anxiety, depression and demoralisation.
As yet, no clinical trials have been conducted on patients with depression who do not have advanced cancer. But this may be about to change — both Vollenweider and Nutt have approval for phase II clinical trials in patients with depression, which could start imminently.
The uncertainty as to the start date, Nutt says, is because he and his team are still waiting for the final go-ahead from the UK medicines regulator, the London-based Medicines and Healthcare products Regulatory Agency (MHRA). And it has already taken almost two years to get to this stage.
The reason that the trial has taken so long to get off the ground is because magic mushrooms were made illegal in 2005, with psilocybin classified as a schedule 1 drug, which means it has a high potential for abuse and no accepted medical use. According to Nutt, this crackdown was triggered after people figured out how to freeze-dry the mushrooms while maintaining their psychedelic properties; previously they had to be eaten fresh to have an effect. “Shops in Camden started selling them and the Daily Mail ran a campaign to get them shut down. After this, the mushrooms were banned with no consultation, it was a politically motivated show of strength,” he says.
Running into regulation
Conducting clinical research using schedule 1 drugs is difficult in the UK and the United States. Both the Johns Hopkins and NYU teams struggled to get their psilocybin trials started because of regulatory hurdles. Grob says that it took a couple of years to get a licence for the anxiety trial and that the psilocybin has to be “in a safe, bolted to a wall, in a locked room within another locked room”. Johnson adds that it took nearly a year to get approval for his first psilocybin trial; even then, he says, maintaining approval is hard. “Because of its status as having no medical use it is very hard to get a licence to use psilocybin in clinical trials, but without clinical trials we will never establish a medical use.”
“Because of its status as having no medical use it is very hard to get a licence to use psilocybin in clinical trials, but without clinical trials we will never establish a medical use.”
The stringent regulatory requirements also make it costly to research schedule 1 drugs because “everyone needs a licence: the manufacturer, the hospitals, the researchers”, says Nutt. “It is ten times more expensive to use these drugs compared to others that are not schedule 1.” In turn, this makes securing enough funding for clinical trials, never easy, harder than usual. To add to this, pharmaceutical companies are largely uninterested in schedule 1 drugs and government organisations are reluctant to support the research. To fill this void, the Heffter Research Institute was founded in Santa Fe, New Mexico in 1993 by a number of eminent scientists, including Grob. It specialises in funding research into hallucinogens. Similarly, the Beckley Foundation in Oxford funds “consciousness and drug policy research” and both organisations have contributed to a number of the studies into psilocybin, including the tobacco addiction trial at Johns Hopkins.
However, Johnson says that the US Food and Drug Administration (FDA) in Silver Spring, Maryland, has been supportive of the work at Johns Hopkins. In the US, the FDA approves medicines for use and the Drug Enforcement Administration (DEA), in Springfield, Virginia, among other bodies, decides on drug scheduling and is in charge of policing their use. “The FDA is very scientifically driven, it has done an extraordinary job in supporting our work — the data really do suggest this is worthy of investigation,” says Johnson.
According to a DEA spokesperson, if psilocybin can get medicinal approval from the FDA then the DEA is obliged to down-schedule the drug (Johnson believes the data relevant to scheduling criteria suggest psilocybin should be schedule “3 or 4”). However, the spokesperson says that rescheduling is a multi-agency process that can take a number of years and, until a final decision has been reached, researchers will still have to abide by the schedule 1 rules — even if psilocybin did get its FDA license.
In the UK, the scheduling rules are slightly different. Either the MHRA or the European Medicines Agency, also based in London, is responsible for approving drugs for medical use, while the Home Office decides the schedule. But, unlike the US, medical approval is no guarantee that a schedule 1 drug will be reclassified. A Home Office spokesperson says that if there is evidence of a medical use, then the Home Office has to ask the Advisory Council on the Misuse of Drugs to perform a consultation, but there is no obligation to take the drug out of schedule 1.
Nutt, who was previously chair of the ACMD but was fired in 2009 because of his stance on drugs, is damning of the UK’s drugs laws, saying that the schedule 1 classification of many potentially useful compounds “is the biggest censorship of life sciences research in human history”.
“Schedule 1 classification of many potentially useful compounds ‘is the biggest censorship of life sciences research in human history.’”
Heroin and cocaine are both schedule 2 drugs, owing to their use in medicine, which means hospitals and universities do not need a licence to research them and the process is much easier and cheaper, despite scoring at the top end of the scale for harm to users and society. In comparison, Nutt says, psilocybin “scores the lowest on the physical and social harm scale of any drug that is misused”.
Safety and acceptability
But psilocybin is not completely free from side-effects. “Some people get modest short-lived increases in blood pressure, heart rate, headaches and a number report subjective effects such as fear and anxiety, but these are readily managed in a clinical setting,” says Johnson. None of these adverse events were reported as “clinically significant” during the addiction trial. But Johnson admits that fears people could do dangerous things while taking psychedelics recreationally — such as panicking in risky situations or having accidents — are not unfounded. Nonetheless, he believes that at a population level the risks are very low and that they are easily managed in the clinic. And, he adds, long-term follow-up research has found no significant adverse outcomes over a year after psilocybin sessions.
Historically, there have been concerns over whether psychedelics could increase the risk of schizophrenia. Vollenweider explains that the pattern of brain activity with psychedelics does mimic that seen in the early stages of schizophrenia but not in the chronic stages of the disease. Despite this, he does not believe that psychedelics will cause the disease, although they could provoke it.
According to two large surveys from the 1960s and 1970s, only about 0.1–0.2% of subjects who have been given LSD in a controlled, clinical setting showed prolonged psychosis-like reactions, says Vollenweider[7]
[8]
. “There is evidence that some subjects who became schizophrenic were polydrug abusers or had schizophrenia in first degree relatives.” In the general population about 1% of people have schizophrenia. “We need to carefully select patients who do not have a history [of schizophrenia] in the family.”
Vollenweider emphasises that there have been no cases of prolonged psychosis or precipitation of schizophrenia reported in recent studies of psilocybin, even after 16 months of follow up[9]
.
Moving forwards, he hopes that research into psilocybin is just the beginning, and that it leads to the discovery of other, more effective analogues, which would have fewer stigmas attached to them. “In the end what counts is whether it really helps,” he says.
Johnson also agrees that “psychedelics are not for everyone” but says that many people could be helped. “It is not just applicable to smoking — it could also treat other drug addictions, and non-drug disorders like gambling addiction and eating disorders too.” He imagines that in the future specialist clinics could be set up with trained therapists administering the treatments. “They won’t be given to patients to take home.”
But a major question is whether society will be comfortable with the use of psychedelics in medicine after decades in which they have been socially unacceptable. “In the 1960s one-time Harvard researcher Tim Leary famously told every teenager to take LSD, which was very irresponsible and caused a lot of social trauma,” Johnson says. He believes that as research into psychedelics continues, they will become more accepted. “It is a lot more like traditional medicine than people think,” says Johnson. “Eventually these drugs will become boring.”
References
[1] Johnson M, Garcia-Romeu A & Cosimano MP. Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. Journal of Psychopharmacology. 2014;28(11):983–992.
[2] Krebs TS & Johansen P-Ø. Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials. Journal of Psychopharmacology. 2012;26(7):994–1002.
[3] Schenberg EE et al. Treating drug dependence with the aid of ibogaine: A retrospective study. Journal of Psychpharmacology 2014;28:993–1000.
[4] Carhart-Harris RL, Erritzoe D & Williams T. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. PNAS . 2012;109:2138–2143.
[5] Kraehenmann R, Preller KH & Scheidegger M. Psilocybin-induced decrease in amygdala reactivity correlates with enhanced positive mood in healthy volunteers. Biological Psychiatry 2014; doi: 10.1016/j.biopsych.2014.04.010.
[6] Grob C, Danforth AL & Chopra GS. Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Archives of General Psychiatry 2011;68:71–78.
[7] Cohen S. Lysergic acid diethylamide: Side effects and complications. Journal of Nervous and Mental Disease 1960;130:30–40.
[8] Malleson N. Acute adverse reactions to LSD in clinical and experimental use in the United Kingdom. British Journal of Psychiatry 1971;118:229–230.
[9] Studerus E, Kometer M & Hasler F. Acute, subacute and long-term subjective effects of psilocybin in healthy humans: a pooled analysis of experimental studies. Journal of Psychopharmacology 2011;25:1434–1452.
You may also be interested in

Voice of the Voiceless: co-produced materials to help reduce stigma for people receiving opioid substitution treatment in pharmacies

PJ view: The government’s approach to illegal street drugs should focus on treatment and prevention
