NIBSC / Science Photo Library
Only one man has been cured of HIV, Timothy Ray Brown. The American, then living in Berlin, contracted the virus in 1995 and managed it well with medication. But in 2006 he was diagnosed with acute myeloid leukaemia, a cancer of white blood cells generated in the bone marrow. Brown had to undergo a gruelling regimen of chemotherapy to eradicate the cancerous cells from his body and later received a bone marrow transplant.
This was when Brown’s luck changed. He was under the care of Gero Hütter, who was working at the Benjamin Franklin Hospital in Berlin at the time. “Dr Hütter was very astute… he found a bone marrow donor whose cells were immune to HIV,” explains Janet Siliciano, infectious diseases researcher at Johns Hopkins University, Maryland. After the bone marrow transplant, Brown’s immune system was resistant to HIV infection. The virus has never returned[1]
.

Source: Courtesy of Janet Siliciano
Janet Siliciano, infectious diseases researcher at Johns Hopkins University, says the case of Timothy Ray Brown generated a huge outpouring of funding for research into a cure for HIV infection
Before this singular success story was announced in 2008, most researchers did not have much hope of finding a cure, says Siliciano. But now “the field has changed dramatically”, she says, explaining that it’s generated a huge outpouring of funding for research into a cure for HIV infection. In 2016 alone, the National Institutes of Health (NIH) in the United States has announced an investment of US$30m to fund six research projects for five years. The overall NIH budget for HIV research is US$3bn.
Of course, giving all 37 million people infected with HIV a bone marrow transplant from a donor that is immune to the infection is neither feasible nor desirable, especially considering that HIV medication is extremely effective at limiting symptoms. These drugs reduce viral replication to undetectable levels, but do not cure the infection, meaning patients must follow a daily regimen for the rest of their lives. The drugs also come with a risk of side effects, such as kidney and liver problems, and even nerve damage. So researchers and pharmaceutical companies have ramped up efforts to find a way to replicate the outcome achieved with Brown, without such an invasive method.
But first, they must find a way to drain the viral reservoir.
Hide and seek
The existence of the viral reservoir was first discovered in 1997. There had been incredible progress in treating HIV in the previous two years. This was the time that the potent cocktail of drugs called combined antiretroviral therapy (cART) first became available to patients, changing the disease from deadly to manageable.
It was hypothesised that it might take only two to three years to cure an infected individual of HIV
Early research on the drugs showed that the amount of virus in the blood plummeted by 99% just two weeks after starting treatment, followed by a further steady, but slower, decline[2]
. Based on this, it was “hypothesised that it might take only two to three years to cure an infected individual of HIV”, says Siliciano.
But this turned out not to be the case. Despite the fact that there are now “an extraordinary number of cART drugs that stop the virus replicating”, says Silicano, even after a patient has been taking them for years, once they stop taking them the virus will rebound in a matter of weeks. In 1997, Siliciano’s husband, Robert Siliciano, who leads the HIV reservoir research group at Johns Hopkins, confirmed what he had suspected. Inside certain long-lived immune cells, HIV stopped replicating and was able to hide[3]
.
The pool of these silent HIV-infected cells is the latent reservoir. They are “present in everybody with HIV infection. The immune system doesn’t see them and the drugs don’t affect them. They are the major barrier to curing HIV infection”, says his wife Siliciano.
HIV mainly infects and replicates in cells called CD4+ T cells, or “helper” T cells. These cells are an essential part of the immune system. Ordinarily, when HIV replicates inside the activated CD4+ T cells it kills them. But sometimes HIV does not kill the CD4+ T cells and some of them, as part of a natural immune process, can change from being an activated cell to a resting, “memory” cell. Memory cells allow for future responses to the same foreign pathogen. HIV ceases to replicate inside these memory CD4+ resting cells. HIV integrates its genome into the DNA of the host cell and becomes invisible to the immune system as the CD4+ T cell is returning back to a resting state.
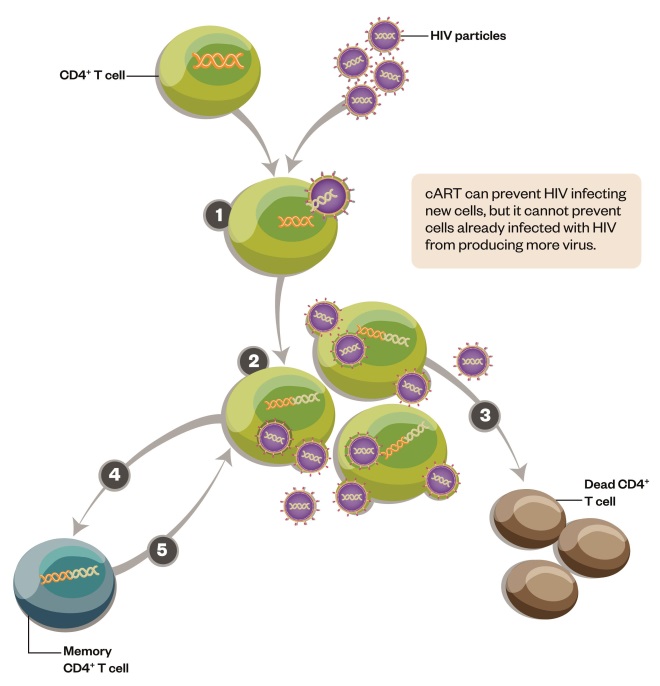
Figure 1: HIV replication and formation of the latent reservoir
HIV primarily infects CD4+ T cells of the immune system. Replication of the virus inside the cells usually kills them. But sometimes the CD4+ T cells don’t die and instead become memory cells. HIV can lie dormant inside these memory cells for many years, creating a reservoir of infection that is invisible to antiviral treatment and to the immune system.
1) HIV infects activated CD4+ T cells and integrates its genome into the host DNA.
2) HIV replicates within the CD4+ T cell and infectious virions are released.
3) Most infected CD4+ T cells die following viral replication.
4) A tiny number of CD4+ T cells survive and become resting, memory cells. HIV is integrated into the cell’s DNA, but stops actively replicating.
5) These latently infected cells can live for many years and are not affected by antiviral drugs or by the immune system. The cells can be reactivated in response to appropriate antigen.
For years, researchers thought “we’re not going to be able to do anything about [the latent reservoir]”, says Janet Siliciano. But they were comforted by the fact that there is “really great” antiretroviral treatment, she says.
It went from oh there’s nothing we can do about it, to oh look there’s a cure, let’s see if we can replicate it
And then, with the cure of Brown, “it went from oh there’s nothing we can do about it, to oh look there’s a cure, let’s see if we can replicate it”, says Janet Siliciano. That really was a driver for all these cure research studies, she adds, since “it’s given people a ton of ideas [of] how to reduce the reservoir”. Siliciano and her husband are now involved in multiple NIH-funded collaborations with other research institutes.
Kick and kill
For those intent on finding a cure for HIV, most research is focused on a “kick and kill” approach. The first step is to reawaken the HIV virus inside the memory CD4+ T cells — the “kick”. This makes the infected cells visible to the immune system so that it can carry out the next part — the “kill”.
One clinical trial made the headlines in the UK after the first patient completed treatment with the kick and kill approach[4]
. It is reportedly the first time this strategy has been tested in humans. “We’re looking to see if there’s a way to eradicate the virus from the body,” says Mark Samuels, managing director of the NIHR Office for Clinical Research Infrastructure (NOCRI), which is funding and coordinating the research.

Source: Courtesy of Mark Samuels
Mark Samuels is managing director of the NIHR Office for Clinical Research Infrastructure, which is funding and co-ordinating a study that is using the “kick and kill” approach to try to eradicate HIV
In total, 52 patients will be recruited to complete the protocol. All of them will receive four-drug cART to control replication of the virus, but one half of the participants will then receive two vaccines. The vaccines are designed to train the immune system to target a part of the HIV virus that rarely elicits a natural immune response, but that is critical to the survival of the virus. This means that it is unlikely that this part of the virus will mutate to evade the immune system.
Next, these patients will receive ten doses of vorinostat, a histone deactylase inhibitor. Histone deacetylases help keep HIV in a silent, latent state. By blocking this, vorinostat is “kicking the virus into life”, says Samuels. The idea is that the immune system, primed by the vaccines, is then able to recognise and kill these infected cells after the virus becomes active. The kill is carried out by killer or CD8+ T cells.
If it works, it might clear the reservoir… which would be fantastic
“If it works, it might clear the reservoir… which would be fantastic,” says Samuels. But at the moment it is too soon to say whether the protocol has been successful. It has been found to be safe and well tolerated, but treatment of the other 51 patients isn’t due to be completed until the end of 2017, and after that participants will require years of monitoring.
Samuels says this is the “most significant attempt in the UK to find a cure for HIV”. The current clinical trial is the culmination of six years work and a collaboration between five universities: Oxford, Cambridge, Imperial College London, King’s College London and University College London.
Toll-like receptor 7 agonists
In the United States, the biopharmaceutical company Gilead, based in Foster City, California, launched a programme that aimed to cure HIV in 2008, the same year that the cure of Brown was announced. The work stemmed from research Gilead was carrying out into hepatitis C. While studying molecules called Toll-like receptor 7 (TLR7) agonists, it “found they may be useful for eliminating the HIV reservoir”, says Romas Geleziunas, director of clinical virology at Gilead.
The TLR7 agonists were found to activate HIV inside resting CD4+ T cells in the laboratory and can also activate simian immunodeficiency virus (SIV), the non-human primate version of HIV. This meant that TLR7 agonists could be a candidate for the “kick” part of the strategy. The company decided to begin trials in monkeys – but there was a catch. The SIV infected monkeys needed to take cART to suppress viral replication and recreate the latent reservoir seen in humans. “But, you know, monkeys don’t like pills,” says Geleziunas, “they spit out pills and refuse to take them”.
For a while, the HIV-cure field did not have an effective animal model. “It took a long time, the field wasn’t able to suppress SIV in the way HIV is suppressed in humans,” says Geleziunas. And so Gilead, which manufactures some of the cART medicines, eventually formulated an injectable version, which recreated the suppression and viral reservoir seen in humans[5]
.
With an animal model finally available, Gilead was able to test its TLR7 agonists. The results, which were reported in 2015, were “particularly interesting”, says Geleziunas. In a group of ten rhesus macaques suppressed on cART, four were given seven bi-weekly escalating doses of the drug that Gilead hoped would activate the SIV. “We saw big spikes of the virus in the bloodstream, which would indicate we had reactivated a substantial reservoir of SIV,” he explains.
“This is what the whole field is trying to do,” adds Geleziunas, explaining that Gilead felt it needed to be transferred to humans as quickly as possible. A clinical trial is now under way that is testing a range of doses to see if the viral spikes can be recreated. Results are due in early 2017.
However, hope that the TLR7 agonist might have eradicated the reservoir in macaques was premature. After stopping cART, all of the monkeys rebounded, albeit they now had 30–90% lower levels of virus in their blood than before[6]
. So Gilead took a slightly different approach in its next animal trial with the human drug candidate GS9260. The macaques received 10–19 lower doses. Some showed spikes in the level of virus in the bloodstream and some didn’t. But for two of the nine macaques, the virus never returned. “This looks like a functional cure,” says Geleziunas tentatively[7]
. This would mean that the virus can be kept under control without ART for years or decades.
Gilead is examining the two macaques to figure out what makes them different to the other seven macaques. These monkeys have either cleared the reservoir or have a superior immune response that can control it as a result of the activation, or both, says Geleziunas.
However, the company doesn’t fully understand how the TLR7 agonists are reactivating HIV and SIV. TLR7 is present in certain cells of the immune system, but not CD4+ T cells. The receptor is activated in response to single stranded RNA, which is often a hallmark of viruses. So, TLR7 agonists mimic this viral RNA, says Geleziunas. But “we don’t quite know yet how it activates HIV in other CD4+ T cells”, he adds.
Our mission is to keep pushing the frontiers back… it’s clear that if there was a cure for HIV that would be better than patients having to take cART
Janet Siliciano says that Gilead’s programme looks promising. But she adds that it remains to be seen if the agonists can lead to the death of the reservoir cells.
To this end, Gilead is planning future studies that combine TLR7 agonists with an antibody therapy to enhance the kill response of the immune system. These are broadly neutralising antibodies that can target most strains of HIV and recruit CD8+ T killer cells.
Gilead has also launched a cure grants programme offering US$2.5m per grant for academic, charity or community projects involved in HIV cure work. Geleziunas says that the grants are completely philanthropic: “Our mission is to keep pushing the frontiers back… it’s clear that if there was a cure for HIV that would be better than patients having to take cART.”
DART antibodies
Another approach Gilead has collaborated on is dual-affinity re-targeting (DART), a technology developed by Macrogenics, a biotechnology company based in Rockville, Maryland, which now leads the research alone. “Our technology can design antibodies that have dual specificity,” says Scott Koenig, chief executive officer and co-founder of Macrogenics. One arm of the antibody is designed to recognise a specified target while the other arm acts as a trigger for killer T cells.

Source: Courtesy of Scott Koenig
Scott Koenig is chief executive officer and co-founder of Macrogenics, a biotechnology company that is designing dual specificity antibodies that can target the latent reservoir of HIV
Ordinarily, killer T cells each recognise only one specific antigen. But DART negates the need for this unique recognition and directly activates any passing killer cell. The technology to create DARTs was developed around ten years ago, initially for cancer and autoimmune diseases. The company has now expanded their use to infectious diseases and for the past three years has been working on a DART antibody that can target the latent reservoir of HIV. “The opportunity with this technology is that the killer T cells that are engaged aren’t necessarily HIV specific, they just need to be cytolytic [cell killing],” explains Koenig.
The opportunity with this technology is that the killer T cells that are engaged aren’t necessarily HIV specific, they just need to be cytolytic [cell killing]
Its most advanced HIV DART has one arm that recognises a protein that is present on the surface of CD4+ T cells infected with the virus, even when the virus is not actively replicating. In the laboratory, the DART molecule, combined with killer T cells, reduced the pool of latently infected CD4+ T cells.
The strategy has caught the attention of the NIH, which has awarded the company a contract of up to US$24.5m. Macrogenics now has a phase I study planned for 2017.
The DART molecules have already been trialled in humans for other diseases, such as cancer, but this will be a first for patients with HIV. Geleziunas says the research is “very interesting” but cautions that there can be safety concerns when it comes to activating killer T cells.
Creating a vaccination
In Évry, France, a company is developing a strategy that bypasses killer T cells. InnaVirVax is aiming to create a functional cure using a vaccine.
So far, there have been no successful attempts to create a vaccine to either prevent or treat HIV. But Joël Crouzet, chief scientific officer and founder of InnaVirVax, says its vaccine is “totally different to other vaccines”. Researchers in Paris identified an HIV protein that both facilitates viral entry into CD4+ T cells and also triggers the death of these cells at the hands of the immune system[8]
. When the body produces antibodies targeted to this protein, the theory is that it protects CD4+ T cells from infection and from death. “It blocks the mechanism by which HIV affects the immune system,” says Crouzet.

Source: Courtesy of Joël Crouzet
Joël Crouzet, chief scientific officer and founder of InnaVirVax, is developing a vaccine that blocks the mechanism by which HIV affects the immune system
InnaVirVax was founded in 2008, based on this research, and the vaccine that it first developed reached humans in 2012. The vaccine is now in a phase II study, which is designed to select the right dose, but the company has seen encouraging signs from the early stage trial. “We’ve found some activity, CD4+ T cells are going up,” says Crouzet. The results of the trial will be officially reported in the first half of 2017. “This is a new therapeutic paradigm for patients,” says Crouzet. “It’s a very exciting time to be working in this area”. But even if it all goes well, Crouzet says a product won’t be on the market until at least 2022.
So, realistically, will the field be able to come up with a cure? “I really hope so,” says Janet Siliciano. “We’re learning so much about the latent reservoir. There are so many good scientists working on this but it’s a really difficult problem.”
Romas concurs, adding that “ten years ago there was not much mention of a cure strategy, but now the ideas are actually becoming tangible”.
References
[1] Hütter G, Nowak D, Mossner M et al. Long-term control of HIV by CCR5 delta32/delta32 stem-cell transplantation. N Engl J Med 2009;360:692–698. doi: 10.1056/NEJMoa0802905
[2] Perelson AS, Essunger P, Cao Y et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 1997;387:188–191. doi: 10.1038/387188a0
[3] Finzi D, Hermankova M, Pierson T et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295
[4] Imperial College London. Research in viral eradication of HIV reservoirs (RIVER). In: ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Available from: https://clinicaltrials.gov/ct2/show/NCT02336074. NLM Identifier: NCT02336074 (accessed 3 November 2016).
[5] Del Prete GQ, Smedley J, Macallister R et al. Comparative evaluation of coformulated injectable combination antiretroviral therapy regimens in Simian Immunodeficiency Virus-infected rhesus macaques. AIDS Res Hum Retroviruses 2016;32:163–168. doi: 10.1089/AID.2015.0130
[6] Whitney JB, Lim SY, Osuna CE et al. Treatment with a TLR7 agonist induces transient viremia in SIV-infected ART-suppressed monkeys. Conference on Retroviruses and Opportunistic Infections, Seattle, Washington, 23–26 February 2015.
[7] Whitney JB, Lim SY, Osuna CE et al. Repeated TLR7 agonist treatment of SIV+ monkeys on ART can lead to viral remission. Conference on Retroviruses and Opportunistic Infections, Boston, Massachusetts, 22–25 February 2016.
[8] Petitdemange C, Achour A & Dispinseri S. A single amino-acid change in a highly conserved motif of gp41 elicits HIV-1 neutralization and protects against CD4 depletion. Clin Infect Dis 2013;57:745–755. doi: 10.1093/cid/cit335