To view the full infographic, click here
The EMA in London
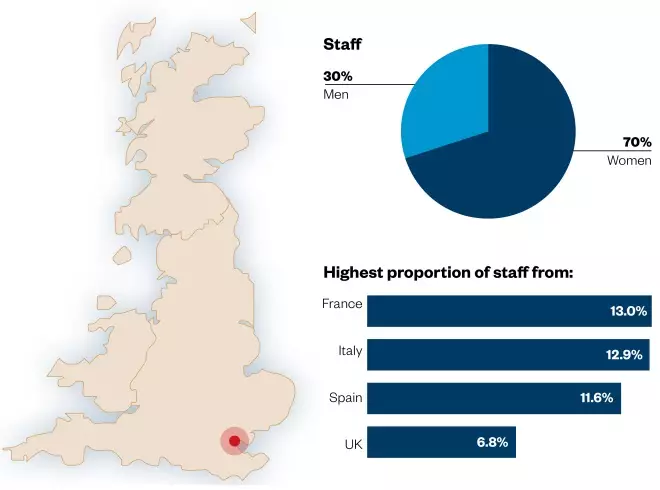
In its first two decades, the EMA recommended the authorisation of a total of 975 human and 188 veterinary medicines. The EMA has almost 900 staff and hosts 36,000 experts for scientific meetings every year. Its total revenue in 2016 was €305,099m.
Assessment of candidate cities and proposed premises
Key for technical requirements:

Layout: space for offices, meeting rooms and archiving facilities
Facilities: IT, security and conference facilities
Relocation plan: proposed timeframe, governance and necessary adaptation work
Candidate cities:
- Amsterdam
- Athens
- Barcelona
- Bonn
- Bratislava
- Brussels
- Bucharest
- Bulgaria
- Copenhagen
- Dublin
- Helsinki
- Lille
- Malta
- Milan
- Porto
- Stockholm
- Vienna
- Warsaw
- Zagreb
The top candidates
| Cities that meet technical requirements | Cities that meet other requirements | Cities that meet all requirements |
|---|---|---|
| Amsterdam | Amsterdam | Amsterdam |
| Barcelona | Barcelona | Barcelona |
| Bratislava | Bonn | Brussels |
| Brussels | Brussels | Copenhagen |
| Copenhagen | Copenhagen | Dublin (Dublin Airport site) |
| Dublin (Dublin Airport site) | Dublin | Milan |
| Milan | Milan | |
| Vienna |
Amsterdam, The Netherlands

Athens, Greece

Barcelona, Spain
Barcelona is one of six cities that meets all of the EMA requirements. But will the October 2017 referendum on Catalan independence from Spain affect the city’s bid?

Bonn, Germany

Bratislava, Slovakia
In addition to meeting the requirements for the premises, Bratislava points out that it should be given priority according to the agreed principle of geographic spread for HQs of EU agencies as it is one of the few EU member states that does not yet host an EU-level agency. However, the city is poorly accessible and an EMA staff survey suggests that fewer than 30% would relocate.

Brussels, Belgium

Bucharest, Romania

Copenhagen, Denmark

Dublin, Ireland
Dublin is proposing three sites for the EMA, only one of which will be ready in time and meets the agency’s technical requirements. However, the city scores high on accessibility, education facilities, and employment and medical care, and at least 65% of staff have indicated they would remain.

Helsinki, Finland

Lille, France

Milan, Italy

Porto, Portugal

Sofia, Bulgaria

Source: Shutterstock.com
No image of the site was provided in the bid presentation.
Stockholm, Sweden
Despite meeting the requirements for accessibility, education facilities, and employment and medical care, Stockholm fails to meet any of the EMA’s technical requirements. The proposed premises will not be ready until Q3 2020, and IT, security and conference facilities have not been addressed in its bid.

Valletta, Malta

Vienna, Austria

Warsaw, Poland

Zagreb, Croatia

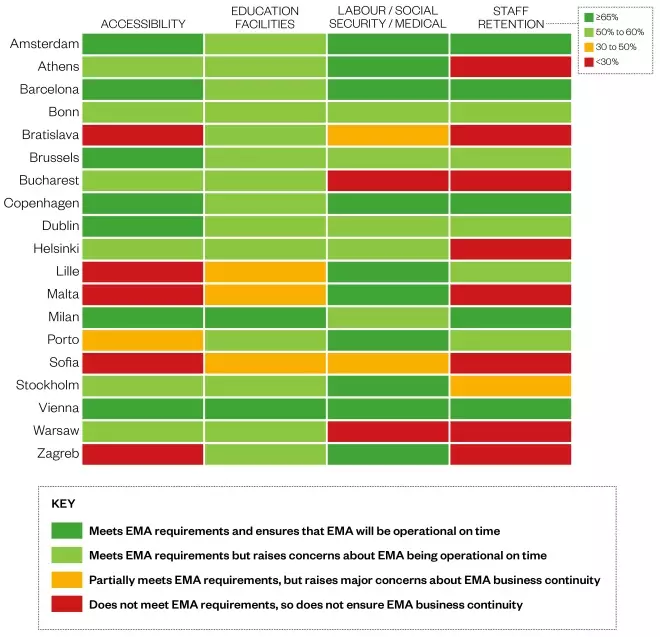
Other requirements
In addition to assessing the candidate cities on their technical ability to host the EMA, the agency assessed them on other requirements, such as accessibility, education facilities, and access to the labour market, social security and healthcare. It also rated the cities on the proportion of current EMA staff that could be expected to stay, based on a staff survey.
References
Sources: EMA annual report 2016; EMA assessment of member states’ bids; candidates’ bids. Images taken from candidates’ bids. Infographic: MAG

