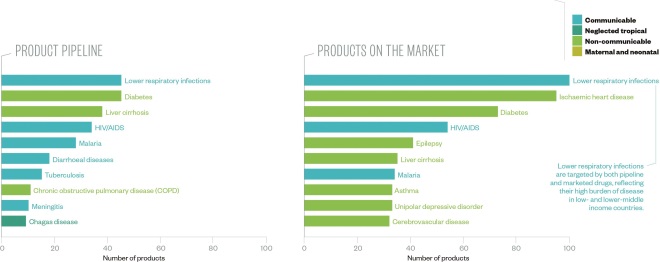
Endemic diseases being targeted
Source: Data and insight, Access to Medicine Index 2014
More than half of the 327 products in development at the 20 largest pharmaceutical companies target five conditions; broadly reflecting the focus of available products, although new treatments are now being developed for several neglected diseases.
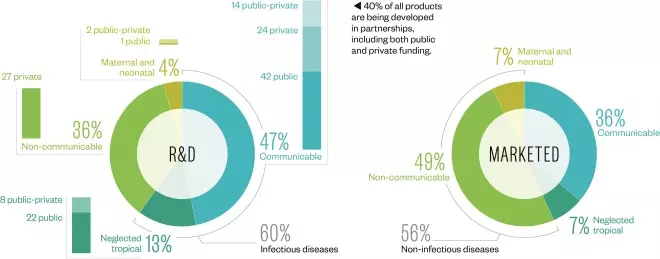
Pipeline and marketed products
Source: Data and insight, Access to Medicine Index 2014
The vast majority of products applicable to developing countries are for infectious diseases. Although there are several drugs for non-communicable diseases in development, most companies struggle to demonstrate how these products will be accessible to patients in developing countries.
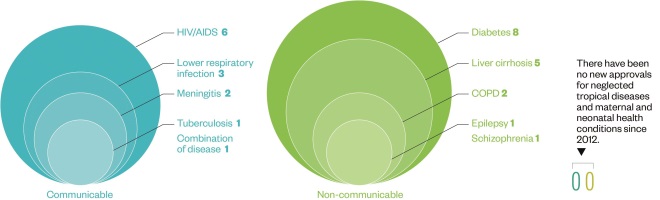
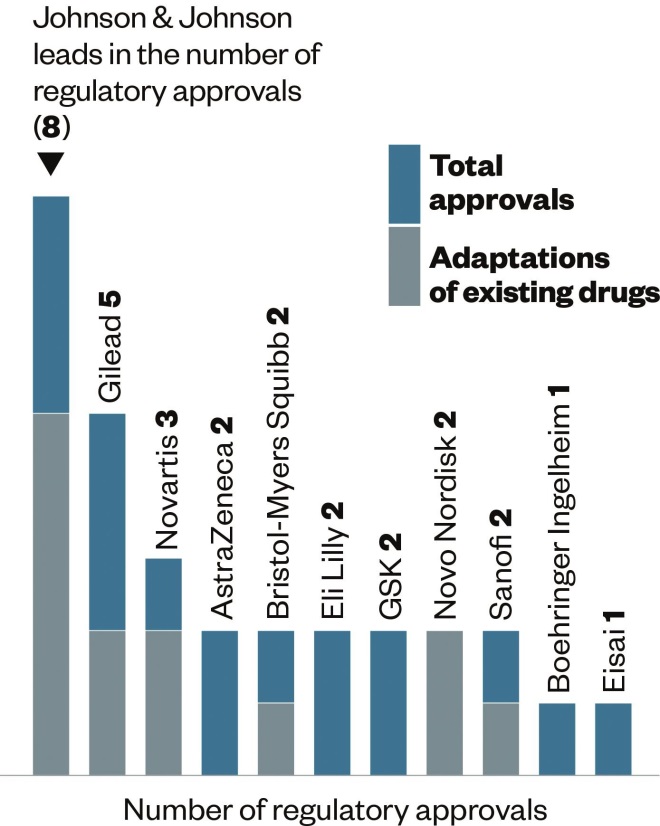
Regulatory approvals
Source: Data and insight, Access to Medicine Index 2014
Since 2012, 11 companies have been granted regulatory approval by the US Food and Drug Administration or the European Medicines Agency for 30 new products for diseases relevant to developing countries
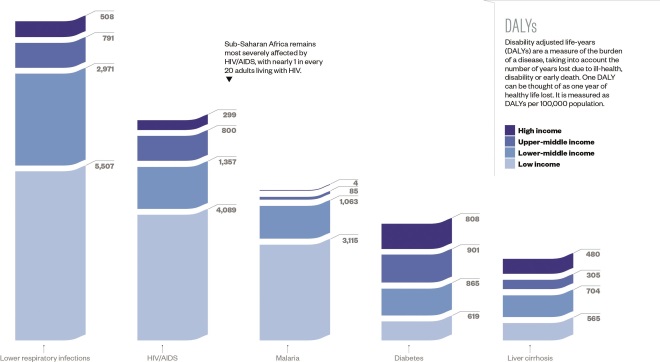
Burden of the top five diseases
Source: World Health Organization
Among these five commonly targeted diseases, the communicable diseases have a higher burden in poorer countries. However, the burden of disease for the non-communicable conditions targeted is shared more equally.
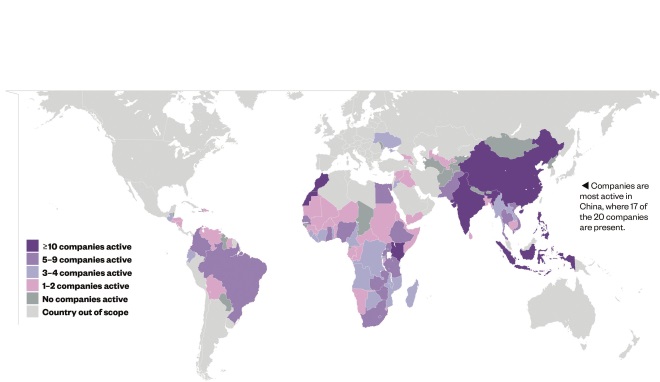
Capacity building
Source: Data and insight, Access to Medicine Index 2014
The 20 largest research-based pharmaceutical companies are active in 75 developing countries in at least one of four areas: research and development; quality management in manufacturing; supply chain management; and pharmacovigilance.
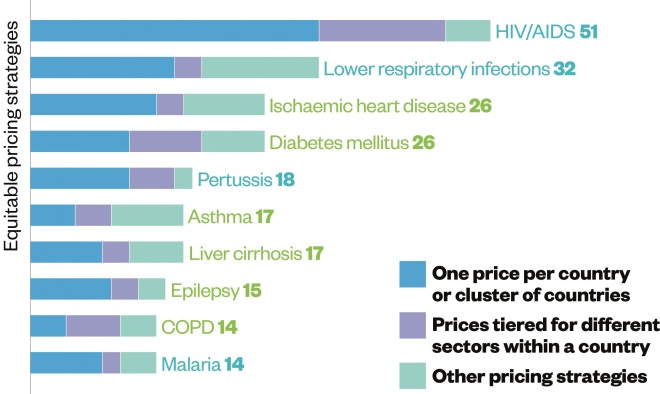
Pricing and donation
Source: Data and insight, Access to Medicine Index 2014
Eighteen of the 20 largest research-based pharmaceutical companies have implemented equitable pricing strategies for developing countries and 15 engage in structured donation programmes.
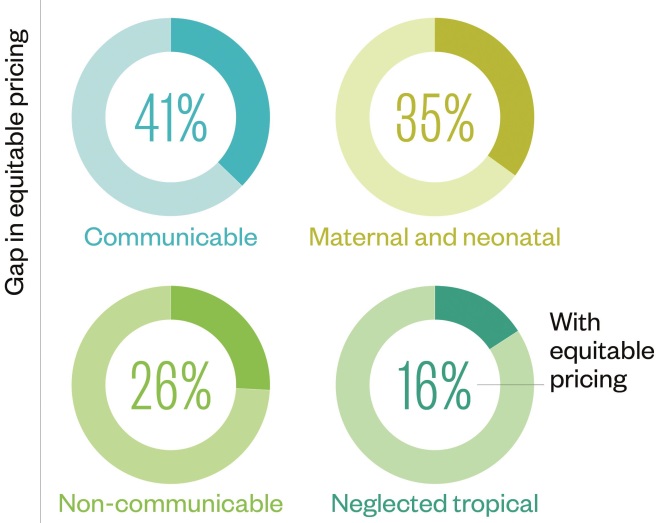
Pricing and donation
Source: Data and insight, Access to Medicine Index 2014
Eighteen of the 20 largest research-based pharmaceutical companies have implemented equitable pricing strategies for developing countries and 15 engage in structured donation programmes.
Pricing and donation
Source: Data and insight, Access to Medicine Index 2014
Eighteen of the 20 largest research-based pharmaceutical companies have implemented equitable pricing strategies for developing countries and 15 engage in structured donation programmes.
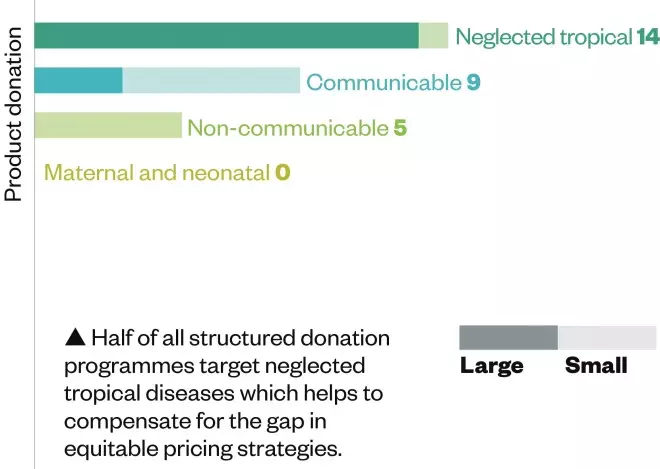
Pricing and donation
Source: Data and insight, Access to Medicine Index 2014
Eighteen of the 20 largest research-based pharmaceutical companies have implemented equitable pricing strategies for developing countries and 15 engage in structured donation programmes.


