STEVE GSCHMEISSNER/SCIENCE PHOTO LIBRARY
The National Institute for Health and Care Excellence (NICE) issued draft guidance on 18 September 2014 recommending that dabrafenib should be available on the NHS for the treatment of melanoma that has spread or can’t be completely removed by surgery in patients with a BRAF gene mutation.
Dabrafenib (Tafinlar) is marketed by GlaxoSmithKline (GSK) and is a biological therapy which works by causing cancer cells with the BRAF V600 mutation to die – slowing or stopping the growth of the melanoma. Around half of patients with melanoma carry this BRAF gene mutation.
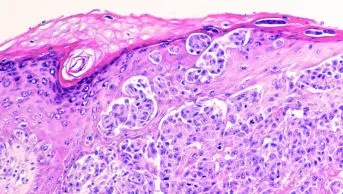
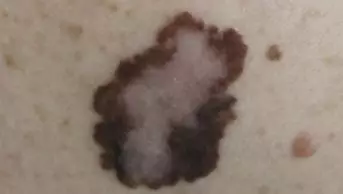
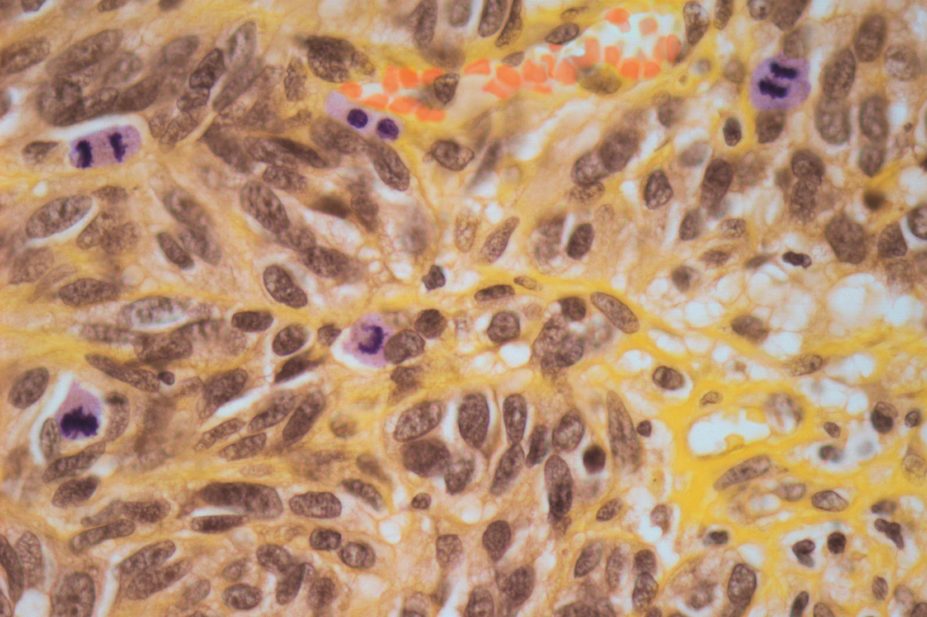
The incidence of melanoma has been increasing steadily since the 1970s due to increased exposure to the sun and use of sunbeds.
“For a long time the treatments available for skin cancer which has spread have been very limited,” said Carole Longson, director for the centre for health technology evaluation at NICE. “However, in recent years a number of breakthrough treatments that can potentially significantly improve the prognosis for some people with malignant melanoma have become available.”
Dabrafenib would be the second melanoma drug targeting the BRAF V600 mutation to be available on the NHS. NICE approved vemurafenib (Roche’s Zelboraf in December 2012. At the same time NICE also approved ipilimumab (Bristol-Myers Squibb’s Yervoy), which is an antibody that activates the body’s immune system to fight melanoma by binding to the cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), a molecule expressed on T-cells.
“The information provided by GlaxoSmithKline, who market the drug [dabrafenib], suggested that dabrafenib works just as well as vemurafenib,” Longson said.
“Drugs like dabrafenib are also thought to have very rapid positive effect for patients, even in those who are very unwell or bed-ridden. In some cases, it has enabled people to resume everyday activities.”
NICE proposes recommending dabrafenib on the basis that GSK provides it to the NHS at a discounted list price. As the committee was able to fully recommend dabrafenib in line with its marketing authorisation, a consultation document was not needed and the recommendations have gone straight to a final draft.