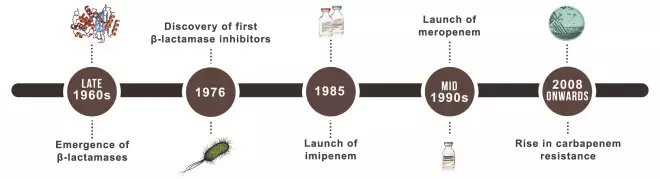
Imipenem and related carbapenem antibiotics are widely seen as the last line in the battle against drug-resistant Gram-negative bacteria. But, as data from many parts of Europe are already showing, that defence is crumbling at an alarming rate.
Between 2009 and 2012, carbapenem resistance in blood culture isolates of Klebsiella pneumoniae in Italy rose from less than 5% to over 25%[1]
. Resistance levels in northern Europe, including the UK, remained low (<1%) during the same period. But, in her annual report for 2013, the Chief Medical Officer for England drew attention to the growing number of isolates of enterobacteriaceae (mainly Klebsiella and Enterobacter spp, and Escherichia coli) in the UK that produced carbapenemases[2]
— enzymes that destroy carbapenems.
“In the UK, there is nothing like the disastrous problem seen in Italy, Greece or India, but numbers of resistant isolates are continuing to rise,” says David Livermore, professor of medical microbiology at the University of East Anglia. “In the whole of 2008, the Public Health England reference lab in London only saw 20 carbapenemase-producing enterobacteriaceae, but now they can expect to confirm 20–30 a week. Some of that is due to better detection, but most is due to a real increase in numbers of cases.”
Simpler times for imipenem
When Merck launched imipenem in the US in December 1985, it was the culmination of two decades of research, triggered by the emergence of beta-lactamase-producing bacteria and the threat of penicillin resistance[3]
.
Like penicillins, carbapenems have a beta-lactam ring, but structural differences confer greater stability against beta-lactamases. Thienamycin was the first carbapenem to demonstrate broad spectrum antibiotic activity but it was chemically unstable, and the closely related imipenem proved more satisfactory.3
Also like penicillins, carbapenems inhibit bacterial cell wall synthesis by acylating penicillin-binding proteins (which catalyse cross-linking of peptidoglycans in the cell wall)3. Early in vitro studies showed that imipenem had the broadest spectrum of activity of the beta-lactam antibiotics available in the early 1980s, and to be active against the beta-lactamase-producing strains and multiresistant bacteria prevalent at that time[4]
.
Because imipenem is deactivated by dehydropeptidase I (DHP-I) in the kidneys, it needs to be administered clinically with the DHP-I inhibitor cilastatin.
In the 1980s, an extensive clinical trial programme with nearly 1,200 patients treated for infections in a wide range of body systems showed an overall efficacy rate of 92% (cured/improved)4. Efficacy ranged from 85% in respiratory infections to 95% in skin and soft tissue and genitourinary infections, and also in septicaemia/endocarditis4. Infecting organisms were Gram positive and Gram negative, aerobic and anaerobic, and in vitro susceptibility to imipenem was 99% (ref.4). This was superior to that of the antibiotics used in comparator trials within the programme, including cefoxatime, moxalactam and combined gentamicin and clindamycin4. Aside from infusion site reactions, the most common drug-related adverse events were nausea, diarrhoea and vomiting4.
“Imipenem arrived at around the same time as the third-generation cephalosporins ceftazidime and ceftriaxone, and, initially, people thought that they were all rather similar. But, over two to three years, it became clear that imipenem wasn’t just another fancy beta-lactam antibiotic and it remained active against enterobacteriaceae and Pseudomonas that became resistant to cephalosporins, including those with AmpC and extended-spectrum beta-lactamases,” recalls Professor Livermore.
Head-to-head with meropenem
In vitro research published a year after the introduction of imipenem suggested that a second carbapenem, meropenem, could be at least as effective[5]
. When administered in the same dosage, meropenem was more active against P. aeruginosa. Both agents had similar activity against Bacteroides spp, but meropenem was more active against Clostridium spp5. It had the added advantage of not requiring co-administration with a DHP-I inhibitor.
Meropenem, launched in Europe in the mid-1990s, has subsequently shown clinical advantages over imipenem[6]
, and it has become the most widely used carbapenem in the UK[7]
.
In a systematic review of 27 randomised controlled trials which compared imipenem with meropenem in severe infections, there was a significantly greater clinical and bacteriologic response with meropenem, a non-significant reduction in deaths and significantly fewer in adverse events6.
Livermore explains that, for 20 years, imipenem held up remarkably well against meropenem. But, in addition to its greater activity against pseudomonas, meropenem has greater dosing flexibility and less potential for causing seizures when used at high doses.
“Meropenem can be used in doses up to 6g per day (2g tds) which is an advantage for the treatment of resistant Pseudomonas, and it penetrates the cerebrospinal fluid so it can be used to treat meningitis. You wouldn’t want to use imipenem at a dose of more than 4g per day (1g qds) and, because of its seizure potential and lack of CSF penetration, it isn’t appropriate for meningitis,” Livermore says.
He explains that newer carbapenems have additional features. Ertapenem has a narrower spectrum and is not active against Pseudomonas and Acinetobacter but its once-daily dosing makes it convenient for home intravenous therapy. Doripenem has a similar profile to meropenem but is more active against Pseudomonas, although it is no longer available in the UK.
Emergence of carbapenemases
By 2000, carbapenems were widely used for Pseudomonas and Acinetobacter infections because of the species’ growing resistance to other antibiotics. They were also used against the growing number of infections due to enterobacteriaceae with extended-spectrum and AmpC beta-lactamases because these confer resistance to cephalosporins but not carbapenems. However, the past decade has seen the spread of bacterial carbapenemases, such as K. pneumoniae carbapenemase (KPC), OXA-48, VIM and New Delhi metallo-beta-lactamase (NDM), which are eroding the sensitivity of enterobacteriaceae to imipenem and other carbapenems.
Some carbapenemases, such as NDM, are spread by plasmids across a wide range of bacteria, whereas KPC is commonly carried by an aggressive clone, K. pneumoniae ST258, which has spread extensively in countries such as Italy, Greece and Israel.
“A single clone is more tractable to infection control and it’s easier for clinical staff to focus on it than on a moving target, such as a carbapenemase carried among different strains and species by a plasmid,” Livermore points out.
A recent analysis of the first 250 patients in the UK whose bacterial isolates produced NDM showed that Klebsiella spp were the source in 55% of cases and E. coli in 25%[8]
. Over half of patients for whom travel history was available had recently been to the Indian sub-continent — demonstrating just how easily bacterial resistance can move across the globe.
In December 2013, Public Health England published a toolkit for the early detection, management and control of carbapenemase-producing enterobacteriaceae. It aims to reduce spread into and within healthcare facilities and residential institutions[9]
.
Livermore believes that early intervention is important if the UK is to control the spread of carbapenemases before it becomes a big problem. “Carbapenems are remarkable antibiotics and, for 20 years until the middle of the past decade, they retained near universal activity against enterobacteriaceae. Even now, with the spread of carbapenemases, imipenem and meropenem are still active against most enterobacteriaceae, so they’ve stood the test of time. I wouldn’t write them off for the future because there’s a good argument for using them in combination with carbapenemase inhibitors. There’s still a lot to play for.”
References
You may also be interested in
The importance of diverse clinical imagery within health education

Government should consider ways to prevent ‘inappropriate overseas prescribing’ of hormone drugs, review recommends
